|
Dithiocarbamates
In organic chemistry, a dithiocarbamate is a chemical compound with the general formula . It contains the functional group with the Chemical structure, structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only one oxygen is replaced the result is thiocarbamate). Dithiocarbamate also refers to the dithiocarbamate ion and its salts. A common example is sodium diethyldithiocarbamate . Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: : Ammonia reacts with carbon disulfide, similarly, to give ammonium dithiocarbamate: : Dithiocarbamate salts are pale colored solids that are soluble in water and Solvent, polar organic solvents. Dithiocarbamic acid A primary amine and carbon disulfide react to give a dithiocarbamic acid: : In the presence of diimides or pyridine, these acids convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
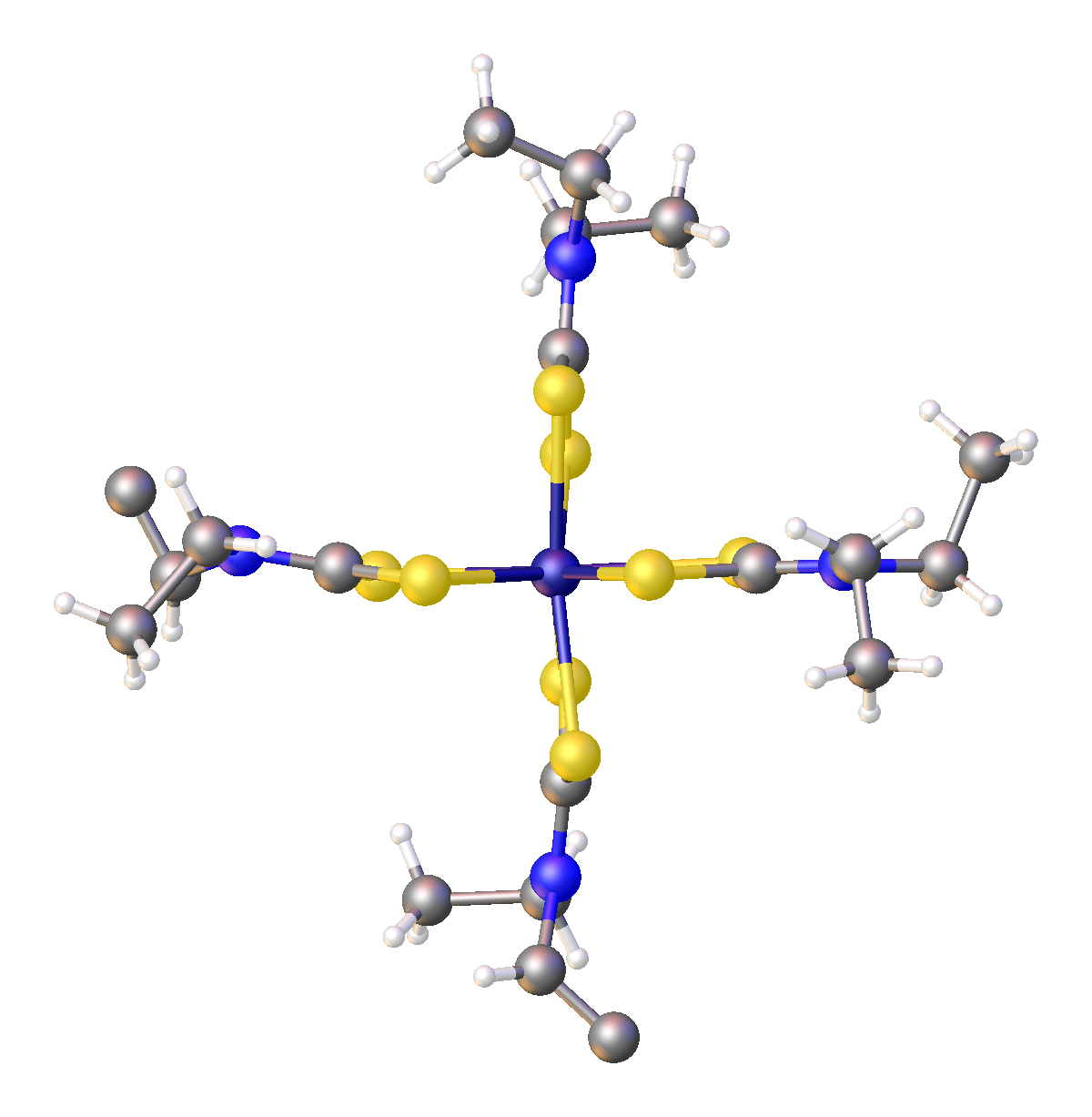
Transition Metal Dithiocarbamate Complexes
193px, Structure of iron tris(diethyldithiocarbamate). Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3. Ligand characteristics Dithiocarbamate anions are bidentate ligands that are classified as L-X ligand in the Covalent bond classification method. In the usual electron counting method, they are three-electron ligands. With respect to HSAB theory, they are classified as soft. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity relative to dithiocarboxylates. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character for the C-N bond. Consequently, barriers to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Diethyldithiocarbamate
Sodium diethyldithiocarbamate is the organosulfur compound with the formula . It is a pale yellow, water soluble salt. Preparation Sodium diethyldithiocarbamate typically crystallizes from water as the trihydrate . The anhydrous salt and the trihydrate are often used interchangeably. Sodium diethyldithiocarbamate is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide: :CS2 + HN(C2H5)2 + NaOH → NaS2CN(C2H5)2 + H2O Other dithiocarbamates can be prepared similarly from secondary amines and carbon disulfide. They are used as chelating agents for transition metal ions and as precursors to herbicides and vulcanization reagents. Reactions Oxidation of sodium diethyldithiocarbamate gives the disulfide, also called a thiuram disulfide (Et = ethyl): : Dithiocarbamates are nucleophiles and thus can be alkylated. Even dichloromethane suffices: : Diethyldithiocarbamate reacts with many metal salts to give transition metal dithiocar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Dimethyldithiocarbamate
Methyl dimethyldithiocarbamate is the organosulfur compound with the formula . It is the one of simplest dithiocarbamic esters. It is a white volatile solid that is poorly soluble in water but soluble in many organic solvents. It was once used as a pesticide. Methyl dimethyldithiocarbamate can be prepared by methylation of salts of dimethyldithiocarbamate: : It can also be prepared by the reaction of a tetramethylthiuram disulfide Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are use ... with methyl Grignard reagents: :{{chem2, CH3)2NC(S)S + CH3MgBr → (CH3)2NC(S)SCH3 + (CH3)2NCS2MgBr References Dithiocarbamates Dimethylamino compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maneb
Maneb (manganese ethylene-bis-dithiocarbamate) is a fungicide and a polymeric complex of manganese with the ethylene bis (dithiocarbamate) anionic ligand. Health effects Exposure to maneb can occur when breathed in; it can irritate the eyes, nose, and throat as well as cause headache, fatigue, nervousness, dizziness, seizures and even unconsciousness. Prolonged or long-term exposure may interfere with the function of the thyroid. Exposure to maneb is also shown to induce a Parkinson's disease like neurotoxicity in mice. It is still challenged whether maneb, along with Paraquat, is an environmental risk factor for Parkinson's disease. Production Manganese(II) ethylenebis(dithiocarbamate) of low ethylenethiourea (ETU) content is prepared by mixing disodium ethylenebis (dithiocarbamate) with formaldehyde in aqueous medium then mixing a water-soluble manganese(II) salt to precipitate the maneb. The product can be further formulated with a metal salt and also with paraforma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Structure
A chemical structure of a molecule is a spatial arrangement of its atoms and their chemical bonds. Its determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together and can be represented using structural formulae and by molecular models; complete electronic structure descriptions include specifying the occupation of a molecule's molecular orbitals. Structure determination can be applied to a range of targets from very simple molecules (e.g., diatomic oxygen or nitrogen) to very complex ones (e.g., such as protein or DNA). Background Theories of chemical structure were first developed by August Kekulé, Archibald Scott Couper, and Aleksandr Butlerov, among others, from about 1858. These theories were first to state that chemical compounds are not a ran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship between given quantities. The plural of ''formula'' can be either ''formulas'' (from the most common English plural noun form) or, under the influence of scientific Latin, ''formulae'' (from the original Latin). In mathematics In mathematics, a formula generally refers to an equation or inequality relating one mathematical expression to another, with the most important ones being mathematical theorems. For example, determining the volume of a sphere requires a significant amount of integral calculus or its geometrical analogue, the method of exhaustion. However, having done this once in terms of some parameter (the radius for example), mathematicians have produced a formula to describe the volume of a sphere in terms of its radius: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent, as a rubber additive, and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese is commonly found in labo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include Polyisobutene, polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crosslink
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. Synthetic polymers : 260px, left, Chemical reactions associated with crosslinking of drying oils, the process that produces curing'' refers to the crosslinking of thermosetting">linoleum. Cros ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |