|
Cream Of Tartar
Potassium bitartrate, also known as potassium hydrogen tartrate, with formula K C4 H5 O6, is the potassium acid salt of tartaric acid (a carboxylic acid)—specifically, l-( + )-tartaric acid. Especially in cooking, it is also known as cream of tartar. It is produced as a byproduct of winemaking by purifying of the precipitate deposited by fermenting must in wine barrels, which arises from the tartaric acid and potassium naturally occurring in grapes. Approved by the FDA as a direct food substance, cream of tartar is used as an additive, stabilizer, pH control agent, antimicrobial agent, processing aid, and thickener in various food products. It is used as a component of baking powders and baking mixes, and is valued for its role in stabilizing egg whites, which enhances the volume and texture of meringues and soufflés. Its acidic properties prevent sugar syrups from crystallizing, aiding in the production of smooth confections such as candies and frostings. When combined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
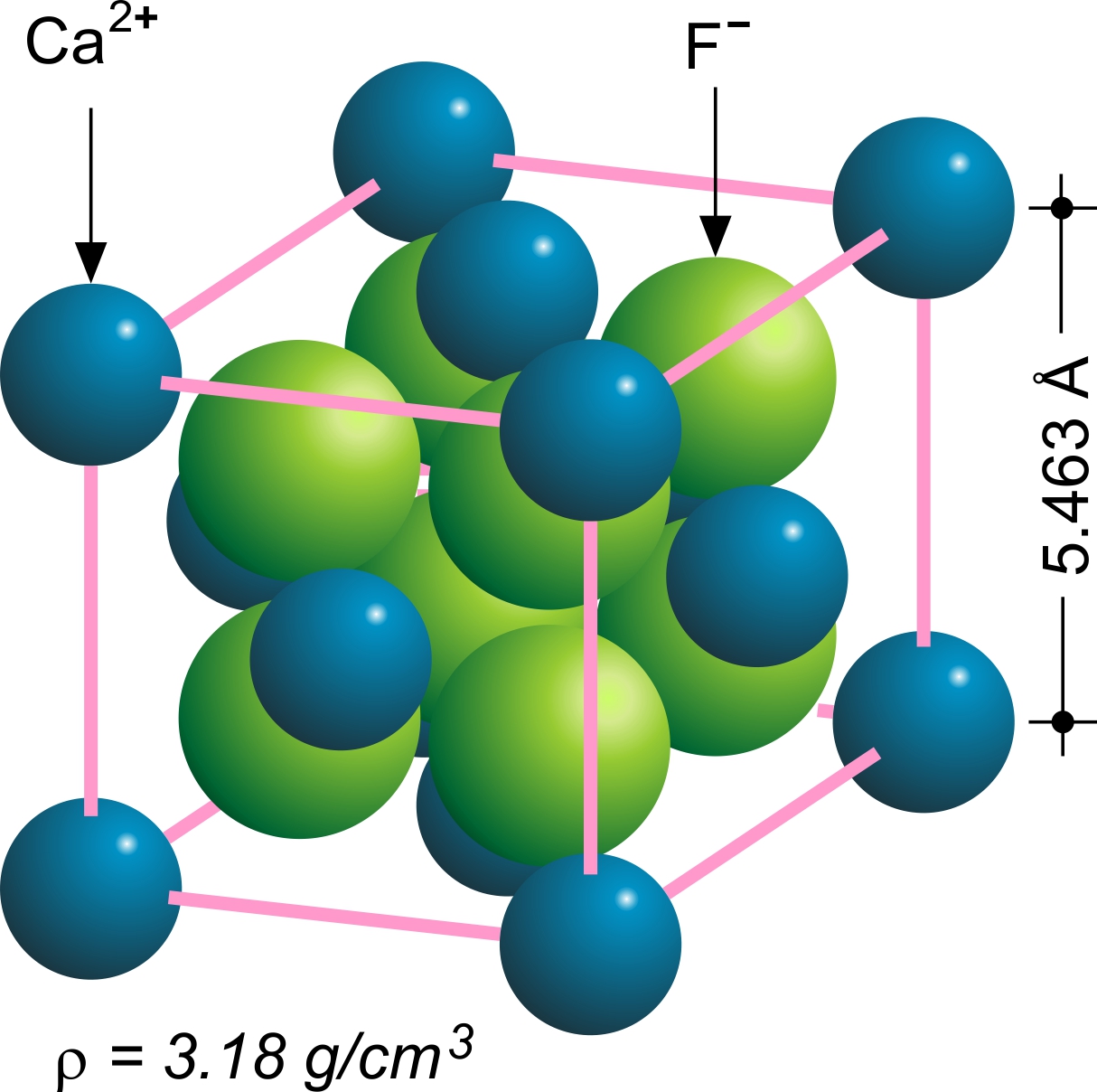
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish Pomerania, German-Swedish pharmaceutical chemist. Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified the elements molybdenum, tungsten, barium, nitrogen, and chlorine, among others. Scheele discovered organic acids Tartaric acid, tartaric, Oxalic acid, oxalic, Uric acid, uric, Lactic acid, lactic, and Citric acid, citric, as well as Hydrofluoric acid, hydrofluoric, Hydrocyanic acid, hydrocyanic, and Arsenic acid, arsenic acids. He preferred speaking German to Swedish his whole life, as German was commonly spoken among Swedish pharmacists.Fors, Hjalmar 2008. "Stepping through Science’s Door: C. W. Scheele, from Pharmacist's Apprentice to Man of Science". Ambix 55: 29–49 Biography Scheele was born in Stralsund, in western Pomerania, which at the time was a Dominions of Sweden, Swedish Dominion inside the Holy Roman Empire. Scheele's father, Joachim (or Jo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Over-the-counter Drug
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) and strengths of final products. The term ''over-the-counter'' (''OTC'') refers to a medication that can be purchased without a medical prescription. In contrast, prescription drugs require a prescription from a doctor or other health care professional and should only be used by the prescribed individual. Some drugs may be legally classified as over-the-counter (i.e. no prescription is required), but may only be dispensed by a pharmacist after an assessment of the patient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diuretic
A diuretic () is any substance that promotes diuresis, the increased production of urine. This includes forced diuresis. A diuretic tablet is sometimes colloquially called a water tablet. There are several categories of diuretics. All diuretics increase the excretion of water from the body, through the kidneys. There exist several classes of diuretic, and each works in a distinct way. Alternatively, an antidiuretic, such as vasopressin ( antidiuretic hormone), is an agent or drug which reduces the excretion of water in urine. Medical uses In medicine, diuretics are used to treat heart failure, liver cirrhosis, hypertension, influenza, water poisoning, and certain kidney diseases. Some diuretics, such as acetazolamide, help to make the urine more alkaline, and are helpful in increasing excretion of substances such as aspirin in cases of overdose or poisoning. Diuretics are sometimes abused by people with an eating disorder, especially people with bulimia nervosa, with the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathartic
In medicine, a cathartic is a substance that ''accelerates'' defecation. This is similar to a laxative, which is a substance that ''eases'' defecation, usually by softening feces. It is possible for a substance to be both a laxative and a cathartic. However, agents such as psyllium seed husks increase the bulk of the feces. Cathartics such as sorbitol, magnesium citrate, magnesium sulfate, or sodium sulfate were previously used as a form of gastrointestinal decontamination following poisoning via ingestion. They are no longer routinely recommended for poisonings. High-dose cathartics may be an effective means of ridding the lower gastrointestinal tract of toxins; however, they carry a risk of electrolyte imbalances and dehydration. Catharsis can be an effect of pesticide poisonings, such as with elemental sulfur Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinegar
Vinegar () is an aqueous solution of diluted acetic acid and trace compounds that may include flavorings. Vinegar typically contains from 5% to 18% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to ethanol using yeast and ethanol to acetic acid using acetic acid bacteria. Many types of vinegar are made, depending on source materials. The product is now mainly used in the culinary arts as a flavorful, acidic cooking ingredient or in pickling. Various types are used as condiments or garnishes, including balsamic vinegar and malt vinegar. As the most easily manufactured mild acid, it has a wide variety of industrial and domestic uses, including functioning as a household cleaner. Etymology The word "vinegar" arrived in Middle English from Old French (''vyn egre''; sour wine), which in turn derives from Latin: (wine) + (neuter gender of , sour). Vinegar was formerly also called . The word "acetic" derives from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartrate
A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. The formula of the tartrate dianion is O−OC-CH(OH)-CH(OH)-COO− or C4H4O62−. The main forms of tartrates used commercially are pure crystalline tartaric acid used as an acidulant in non-alcoholic drinks and foods, cream of tartar used in baking, and Rochelle salt, commonly used in electroplating solutions. As food additives As food additives, tartrates are used as antioxidants, acidity regulators, and emulsifiers. Examples include *sodium tartrates ( E335) **monosodium tartrate ** sodium tartrate ** sodium ammonium tartrate the compound through which Louis Pasteur discovered chirality *potassium tartrates ( E336) ** potassium bitartrate (monopotassium tartrate, cream of tartar) ** potassium tartrate * potassium sodium tartrate ( E337) * calcium tartrate ( E354, used as emulsifier) * stearyl tartrate ( E483, used as emulsifier) In wine In wine, tartrates are the harmless cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |