|
Chalcanthite
Chalcanthite (, ) is a richly colored blue-green water-soluble sulfate mineral . It is commonly found in the late-stage oxidation zones of copper deposits. Due to its ready solubility, chalcanthite is more common in arid regions. Chalcanthite is a hydrate, pentahydrate and the most common member of a group of similar hydrated sulfates, the chalcanthite group. These other sulfates are identical in chemical composition to chalcanthite, with the exception of replacement of the copper ion by either manganese as jokokuite, iron as melanterite, or magnesium as pentahydrite. Other names include ''blue stone'', ''blue vitriol'', and ''copper vitriol''. Uses of chalcanthite As chalcanthite is a copper mineral, it can be used as an ore of copper. However, its ready solubility in water means that it tends to crystallize, dissolve, and recrystallize as crusts over any mine surface in more humid regions. Therefore, chalcanthite is only found in the most arid regions in sufficiently large ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanterite
Melanterite is a mineral form of hydrous iron(II) sulfate: FeSO4·7H2O. It is the iron analogue of the copper sulfate chalcanthite. It alters to siderotil by Dehydration, loss of water. It is a secondary sulfate mineral which forms from the oxidation of primary sulfide minerals such as pyrite and marcasite in the near-surface environment. It often occurs as a ''post mine'' encrustation on old underground mine surfaces. It also occurs in coal and lignite seams exposed to humid air and as a rare Volcanic sublimate, sublimate phase around volcanic fumaroles. Associated minerals include pisanite, chalcanthite, epsomite, pickeringite, halotrichite and other sulfate minerals. It was first described in 1850. Gallery File:Melanterite crystal structure.png, Crystal structure of melanterite File:Cuprian Melanterite - Parys Mountain Mines, Amlwch, Isle of Anglesey, Wales, UK.jpg, Cuprian melanterite References Sulfate minerals Iron(II) minerals Monoclinic minerals Minerals in spa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Sulfate
Copper(II) sulfate is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper,Antoine-François de Fourcroy, tr. by Robert Heron (1796) "Elements of Chemistry, and Natural History: To which is Prefixed the Philosophy of Chemistry". J. Murray and others, Edinburgh. Page 348. and Roman vitriol.Oxford University Press,Roman vitriol, Oxford Living Dictionaries. Accessed on 2016-11-13 It exothermically dissolves in water to give the aquo complex , which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The centers are interconnected by sulfate anions to form chains. Preparation and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal Vein (geology), veins and as secondary minerals in the Redox, oxidizing zone of sulfide mineral deposits. The Chromate ion, chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 **Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O **Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malachite
Malachite () is a copper Carbonate mineral, carbonate hydroxide mineral, with the chemical formula, formula Basic copper carbonate, Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmite, stalagmitic masses, in fractures and deep, underground spaces, where the water table and hydrothermal fluids provide the means for chemical precipitation. Individual crystals are rare, but occur as slender to Acicular (crystal habit), acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur. Etymology and history The stone's name derives (via , , and Middle English ''melochites'') from Greek language, Greek Μολοχίτης λίθος ''molochites lithos'', "mallow-green stone", from μολόχη ''molochē'', variant of μαλάχη ''malāchē'', "mallow". The mineral was given this name due to its resemblance to the leaves of the Malva, mallow plant. Copper (Cu2+) g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcopyrite
Chalcopyrite ( ) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a Mohs scale, hardness of 3.5 to 4 on the Mohs scale. Its Streak (mineralogy), streak is diagnostic as green-tinged black. On exposure to air, chalcopyrite tarnishes to a variety of oxides, hydroxides, and sulfates. Associated copper minerals include the sulfides bornite (Cu5FeS4), chalcocite (Cu2S), covellite (CuS), digenite (Cu9S5); carbonates such as malachite and azurite, and rarely oxides such as cuprite (Cu2O). It is rarely found in association with native copper. Chalcopyrite is a conductor of electricity. Copper can be extracted from chalcopyrite ore using various methods. The two predominant methods are pyrometallurgy and hydrometallurgy, the former being the most commercially viable. Etymology The name chalcopyrite comes from the Greek words , which means cop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. Hydroxide ion The hydroxide ion is naturally produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brochantite
Brochantite is a sulfate mineral, one of a number of cupric sulfates. Its chemical formula is Cu4SO4(OH)6. Formed in arid climates or in rapidly oxidizing copper sulfide deposits, it was named by Armand Lévy (mineralogist), Armand Lévy for his fellow France, Frenchman, geologist and mineralogist André-Jean-François-Marie Brochant de Villiers, A. J. M. Brochant de Villiers. Crystals of brochantite can range from emerald green to black-green to blue-green, and can be acicular or prismatic. Brochantite is often associated with minerals such as malachite, azurite, and chrysocolla, and may form pseudomorphs with these minerals. The mineral is found in a number of locations around the world, notably the southwestern United States (especially Arizona), Serifos in Greece and Chile. Brochantite is a common corrosion product on bronze sculptures located in urban areas, where atmospheric sulfur dioxide (a common pollutant) is present. Brochantite forms mainly in exposed areas where wea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of aragoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropy
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical State of matter, state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are Chemical bond, bonded together in different manners. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a Cubic crystal system, cubic lattice of Tetrahedral molecular geometry, tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for Chemical compound, compounds. The more general term, used for any compound, is Polymorphism (materials science), polymorphism, although its use is usually restricted to solid materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
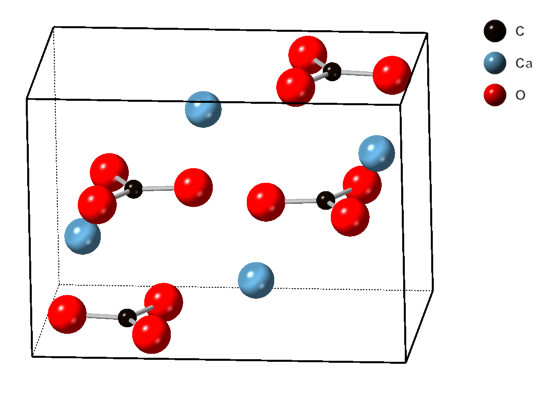
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German , a term from the 19th century that came from the Latin word for Lime (material), lime, (genitive ) with the suffix ''-ite'' used to name minerals. It is thus a Doublet (linguistics), doublet of the word ''wikt:chalk, chalk''. When applied by archaeology, archaeologists and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |