|
Carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains. Organic chemistry Several industrially useful organic chemicals are prepared by carbonylations, which can be highly selective reactions. Carbonylations produce organic carbonyls, i.e., compounds that contain the functional group such as aldehydes (), carboxylic acids () and esters (). Carbonylations are the basis of many types of reactions, including hydroformylation and Reppe reactions. These reactions require metal catalysts, which bind and activate the CO. These processes involve transition metal acyl complexes as intermediates. Much of this theme was developed by Walter Reppe. Hydroformylation Hydroformylation entails the addition of both carbon monoxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
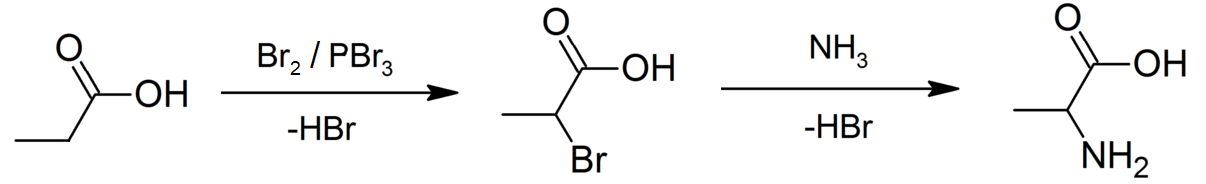
Propionic Acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion as well as the Carboxylate salt, salts and esters of propionic acid are known as propionates or propanoates. About half of the world production of propionic acid is consumed as a preservative for both animal feed and food for human consumption. It is also useful as an intermediate in the production of other chemicals, especially polymers. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas esta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetic Anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of cellulose acetate as well as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air. Structure and properties Acetic anhydride, like most organic acid anhydrides, is a flexible molecule with a nonplanar structure. The C=O and C-O distances are 1.19 and 1.39 Å. The Pi bond, pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole, dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low. Production Acetic anhydride was first synthesized in 1852 by the French chemist Charles Frédéric Gerhardt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Oxidative Carbonylation
Oxidative carbonylation is a class of reactions that use carbon monoxide in combination with an oxidant to generate esters and carbonate esters. These transformations utilize transition metal complexes as homogeneous catalysts. Many of these reactions employ palladium catalysts. Mechanistically, these reactions resemble the Wacker process. Illustrative oxidative carbonylations Oxidative carbonylation, using palladium-based catalysts, allows certain alkenes to be converted into homologated esters: :2 RCH=CH2 + 2 CO + O2 + 2 MeOH → 2 RCH=CHCO2Me + 2 H2O Such reactions are assumed to proceed by the insertion of the alkene into the Pd(II)-CO2Me bond of a metallacarboxylic ester followed by beta-hydride elimination (Me = CH3). Arylboronic acids react with Pd(II) compounds to give Pd(II)-aryl species, which undergo carbonylation to give Pd(II)-C(O)aryl. These benzyl-Pd intermediates are intercepted by alkenes, which insert. Subsequent beta-hydride elimination gives the arylk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dimethyl Oxalate
Dimethyl oxalate is an organic compound with the formula or . It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water. Production Dimethyl oxalate can be obtained by esterification of oxalic acid with methanol using sulfuric acid as a catalyst: :\rm 2\ CH_3OH + (CO_2H)_2\ \xrightarrow\ (CO_2CH_3)_2 + 2\ H_2O Oxidative carbonylation route The preparation by oxidative carbonylation has attracted interest because it requires only C1 precursors: :\rm 4 \ CH_3OH + 4 \ CO + O_2 \xrightarrow\ 2 \ (CO_2CH_3)_2 + 2 \ H_2O The reaction is catalyzed by Pd2+.E. Amadio''Oxidative Carbonylation of Alkanols Catalyzed by Pd(II)-Phosphine Complexes'' PhD Thesis, Ca'Foscari University Venice, 2009 The synthesis gas is mostly obtained from coal or biomass. The oxidation proceeds via dinitrogen trioxide, which is formed according to (1) of nitrogen monoxide and oxygen and then reacts according to (2) with methanol forming methyl nitrite: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Decarbonylation
In chemistry, decarbonylation is a type of organic reaction that involves the loss of carbon monoxide (CO). It is often an undesirable reaction, since it represents a degradation. In the chemistry of metal carbonyls, decarbonylation describes a substitution process, whereby a CO ligand is replaced by another ligand. Organic chemistry In the absence of metal catalysts, decarbonylation (vs decarboxylation) is rarely observed in organic chemistry Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain .... One exception is the decarbonylation of formic acid: :HOH → + The reaction is induced by sulfuric acid, which functions as both a catalyst and a Dehydration reaction, dehydrating agent. Via this reaction, formic acid is occasionally employed as a source of CO in the laboratory in l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are soluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a Functional group#Groups containing oxygen , distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cativa Process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an iridium-containing catalyst, such as the anionic complex diiododicarbonyliridate(i) r(CO)2I2sup>− (1). The Cativa and Monsanto processes are sufficiently similar that they can use the same chemical plant. Initial studies by Monsanto had shown iridium to be less active than rhodium for the carbonylation of methanol. Subsequent research, however, showed that the iridium catalyst could be promoted by ruthenium, and this combination leads to a catalyst that is superior to the rhodium-based systems. The switch from rhodium to iridium also allows the use of less water in the reaction mixture. This change reduces the number of drying columns necessary, decreases formation of by-products, such as propionic acid, and suppresses the water gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |