|
Carbon Dioxide Reforming
Carbon dioxide reforming (also known as dry reforming) is a method of producing synthesis gas (mixtures of hydrogen and carbon monoxide) from the reaction of carbon dioxide with hydrocarbons such as methane with the aid of metal catalysts (typically Ni or Ni alloys). Synthesis gas is conventionally produced via the steam reforming reaction or coal gasification. In recent years, increased concerns on the contribution of greenhouse gases to global warming have increased interest in the replacement of steam as reactant with carbon dioxide. The dry reforming reaction may be represented by: :CH4 + CO2 75^oC2CO + 2H2 Thus, two greenhouse gases are consumed and useful chemical building blocks, hydrogen and carbon monoxide, are produced. A challenge to the commercialization of this process is that the hydrogen that is produced tends to react with carbon dioxide. For example, the following reaction typically proceeds with lower activation energy than the dry reforming reaction itself: :CO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthesis Gas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII (in Germany alone, half a million cars were built or rebuilt to run on wood gas). Production Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. : : : Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of energy: : In principle, but rarely in practice, biomass and related hydrocarbon feedstocks could be used to generate biogas and biochar in waste-to-energy gasification facilities. The gas generated (mostly methane and carbon dioxide) is sometimes described as ''syng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water–gas Shift Reaction
The water–gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen: : CO + H2O CO2 + H2 The water gas shift reaction was discovered by Italian physicist Felice Fontana in 1780. It was not until much later that the industrial value of this reaction was realized. Before the early 20th century, hydrogen was obtained by reacting steam under high pressure with iron to produce iron oxide and hydrogen. With the development of industrial processes that required hydrogen, such as the Haber–Bosch ammonia synthesis, a less expensive and more efficient method of hydrogen production was needed. As a resolution to this problem, the WGSR was combined with the gasification of coal to produce hydrogen. Applications The WGSR is a highly valuable industrial reaction that is used in the manufacture of ammonia, hydrocarbons, methanol, and hydrogen. Its most important application is in conjunction with the conversion of carbon monoxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Processes
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combined without reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be chemically pure. Chemical substances can exist in several different physical states or phases (e.g. solids, liquids, gases, or plasma) without changing their chemical composition. Substances transition between these phases of matter in response to changes in temperature or pressure. Some chemical substances can be combined or converted into new substances by means of chemical reactions. Chemicals that do not possess this ability are said to be inert. Pure water is an example of a chemical substance, with a constant composition of two hydrogen atoms bonded to a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
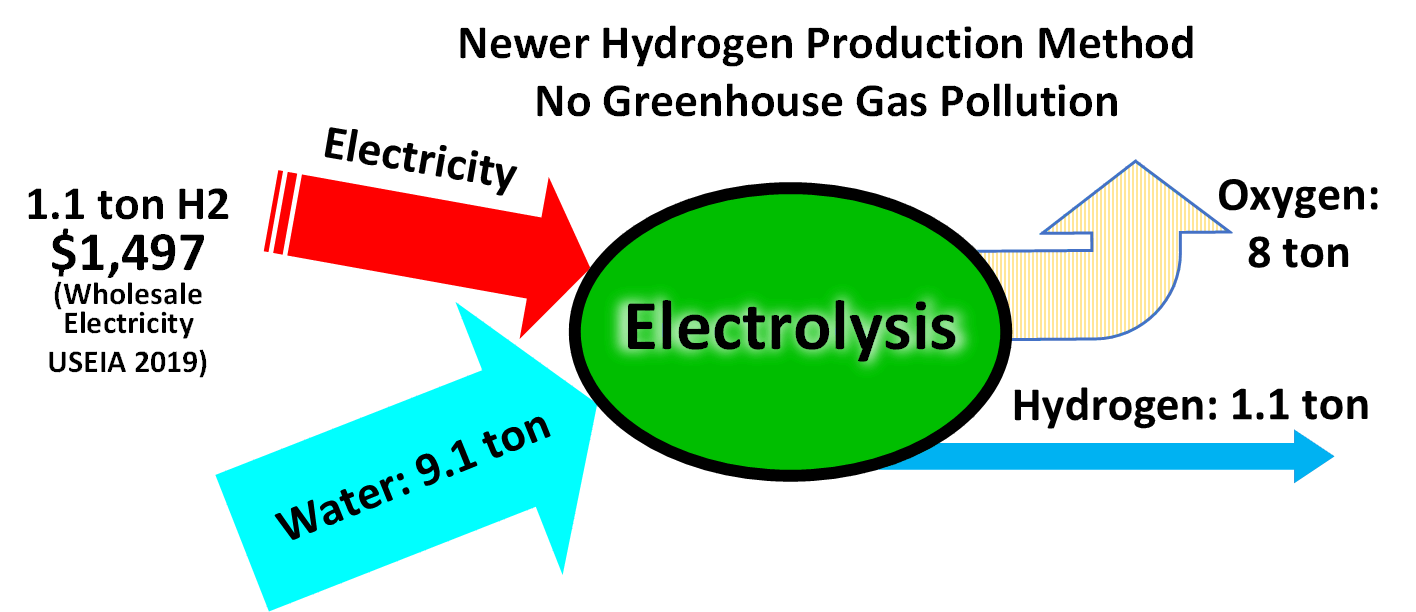
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Capture And Utilization
Carbon capture and storage (CCS) is a process by which carbon dioxide (CO2) from industrial installations is separated before it is released into the atmosphere, then transported to a long-term storage location.IPCC, 2021Annex VII: Glossary atthews, J.B.R., V. Möller, R. van Diemen, J.S. Fuglestvedt, V. Masson-Delmotte, C. Méndez, S. Semenov, A. Reisinger (eds.) IClimate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change[Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, doi:10.1017/9781009157896.022. The CO2 is captured from a large point source pollution, point source, such as a natural gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochar
Biochar is a form of charcoal, sometimes modified, that is intended for organic use, as in soil. It is the lightweight black remnants remaining after the pyrolysis of biomass, consisting of carbon and ashes. Despite its name, biochar is sterile immediately after production and only gains biological life following assisted or incidental exposure to biota. Biochar is defined by the International Biochar Initiative as the "solid material obtained from the thermochemical conversion of biomass in an oxygen-limited environment". Biochar is mainly used in soils to increase soil aeration, reduce soil emissions of greenhouse gases, reduce nutrient leaching, reduce soil acidity, and potentially increase the water content of coarse soils. Biochar application may increase soil fertility and agricultural productivity. However, when applied excessively or made from feedstock unsuitable for the soil type, biochar soil amendments also have the potential for negative effects, including harm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petcoke
Petroleum coke, abbreviated coke, pet coke or petcoke, is a final carbon-rich solid material that derives from oil refining, and is one type of the group of fuels referred to as cokes. Petcoke is the coke that, in particular, derives from a final cracking process—a thermo-based chemical engineering process that splits long chain hydrocarbons of petroleum into shorter chains—that takes place in units termed coker units. (Other types of coke are derived from coal.) Stated succinctly, coke is the "carbonization product of high-boiling hydrocarbon fractions obtained in petroleum processing (heavy residues)". Petcoke is also produced in the production of synthetic crude oil (syncrude) from bitumen extracted from Canada's oil sands and from Venezuela's Orinoco oil sands. In petroleum coker units, residual oils from other distillation processes used in petroleum refining are treated at a high temperature and pressure leaving the petcoke after driving off gases and volatiles, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide (of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as a magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about , rather than the present average of .Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007:Chapter 1: Historical Overview of Climate Change. In:Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. olomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller (eds.) Cambridge University Press, Cambridge, United Kingdom and New Y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |