|
Carbohydrate-binding Module
In molecular biology, a carbohydrate-binding module (CBM) is a protein domain found in carbohydrate-active enzymes (for example glycoside hydrolases). The majority of these domains have carbohydrate-binding activity. Some of these domains are found on cellulosomal scaffoldin proteins. CBMs were previously known as cellulose-binding domains. CBMs are classified into numerous families, based on amino acid sequence similarity. There are currently (June 2011) 64 families of CBM in the CAZy database. CBMs of microbial glycoside hydrolases play a central role in the recycling of photosynthetically fixed carbon through their binding to specific plant structural polysaccharides. CBMs can recognise both crystalline and amorphous cellulose forms. CBMs are the most common non-catalytic modules associated with enzymes active in plant cell-wall hydrolysis. Many putative CBMs have been identified by amino acid sequence alignments but only a few representatives have been s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Alignment
In bioinformatics, a sequence alignment is a way of arranging the sequences of DNA, RNA, or protein to identify regions of similarity that may be a consequence of functional, structural biology, structural, or evolutionary relationships between the sequences. Aligned sequences of nucleotide or amino acid residues are typically represented as rows within a matrix (mathematics), matrix. Gaps are inserted between the Residue (chemistry), residues so that identical or similar characters are aligned in successive columns. Sequence alignments are also used for non-biological sequences such as calculating the Edit distance, distance cost between strings in a natural language, or to display financial data. Interpretation If two sequences in an alignment share a common ancestor, mismatches can be interpreted as point mutations and gaps as indels (that is, insertion or deletion mutations) introduced in one or both lineages in the time since they diverged from one another. In sequence ali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligosaccharides
An oligosaccharide (; ) is a saccharide polymer containing a small number (typically three to ten) of monosaccharides (simple sugars). Oligosaccharides can have many functions including cell recognition and cell adhesion. They are normally present as glycans: oligosaccharide chains are linked to lipids or to compatible amino acid side chains in proteins, by ''N''- or ''O''- glycosidic bonds. ''N''-Linked oligosaccharides are always pentasaccharides attached to asparagine via a beta linkage to the amine nitrogen of the side chain.. Alternately, ''O''-linked oligosaccharides are generally attached to threonine or serine on the alcohol group of the side chain. Not all natural oligosaccharides occur as components of glycoproteins or glycolipids. Some, such as the raffinose series, occur as storage or transport carbohydrates in plants. Others, such as maltodextrins or cellodextrins, result from the microbial breakdown of larger polysaccharides such as starch or cellulose. Gly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
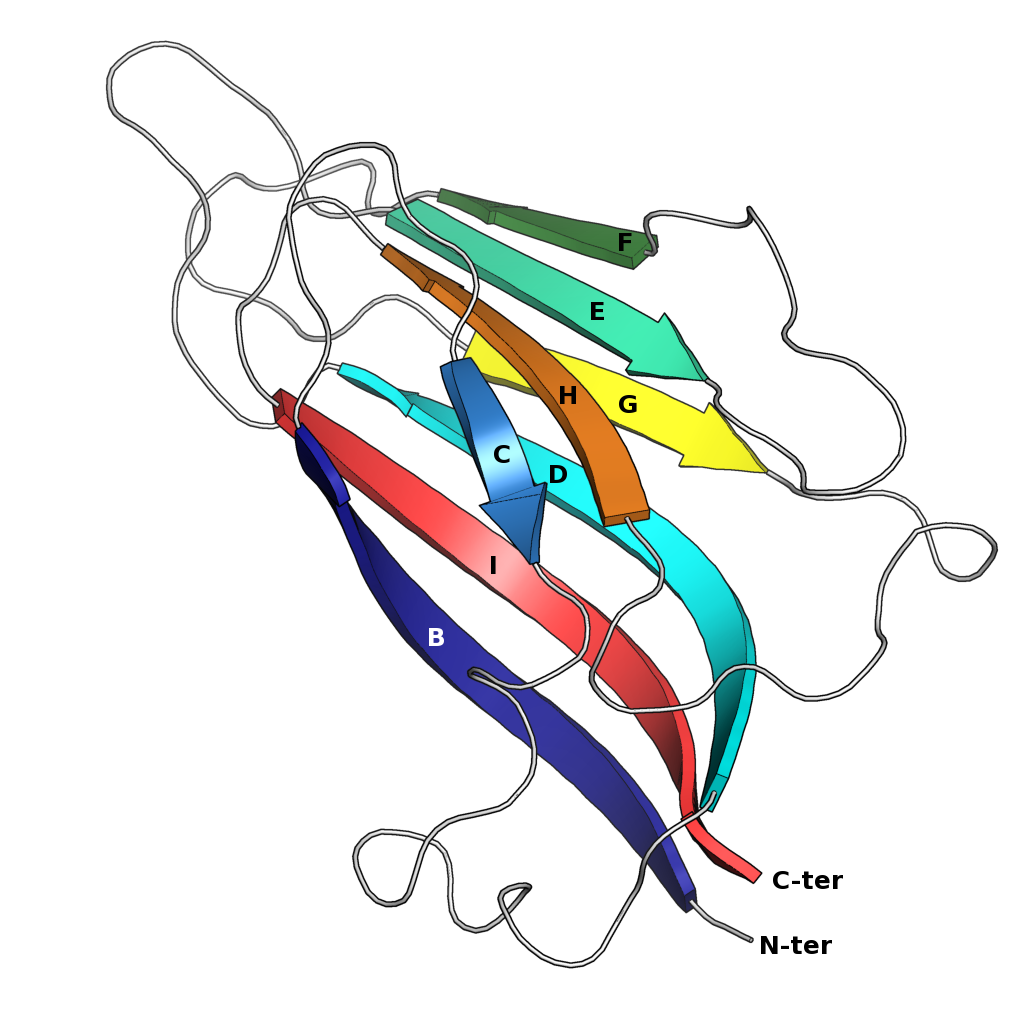
Jelly-roll Fold
The jelly roll or Swiss roll fold is a protein fold or supersecondary structure composed of eight beta strands arranged in two four-stranded sheets. The name of the structure was introduced by Jane S. Richardson in 1981, reflecting its resemblance to the jelly or Swiss roll cake. The fold is an elaboration on the Greek key motif and is sometimes considered a form of beta barrel. It is very common in viral proteins, particularly viral capsid proteins. Taken together, the jelly roll and Greek key structures comprise around 30% of the all-beta proteins annotated in the Structural Classification of Proteins (SCOP) database. Structure The basic jelly roll structure consists of eight beta strands arranged in two four-stranded antiparallel beta sheets which pack together across a hydrophobic interface here citation... uniprot The strands are traditionally labeled B through I for the historical reason that the first solved structure, of a jelly roll capsid protein from the tomato bus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Domain (biology)
In biological taxonomy, a domain ( or ) (Latin: ''regio''), also dominion, superkingdom, realm, or empire, is the highest taxonomic rank of all organisms taken together. It was introduced in the three-domain system of taxonomy devised by Carl Woese, Otto Kandler and Mark Wheelis in 1990. According to the domain system, the tree of life consists of either three domains, Archaea, Bacteria, and Eukarya, or two domains, Archaea and Bacteria, with Eukarya included in Archaea. In the three-domain model, the first two are prokaryotes, single-celled microorganisms without a membrane-bound nucleus. All organisms that have a cell nucleus and other membrane-bound organelles are included in Eukarya and called eukaryotes. Non-cellular life, most notably the viruses, is not included in this system. Alternatives to the three-domain system include the earlier two-empire system (with the empires Prokaryota and Eukaryota), and the eocyte hypothesis (with two domains of Bacteria and A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tertiary Structure
Protein tertiary structure is the three-dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains and the backbone may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure. A number of these structures may bind to each other, forming a quaternary structure. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypeptide chains and amino acid side chains, it was Dorothy Maud Wrinch who incorporated geometry into the prediction of protein structures. Wrinch demonstr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sandwich
Beta-sandwich or β-sandwich domains consisting of 80 to 350 amino acids occur commonly in proteins. They are characterized by two opposing antiparallel beta sheets (β-sheets). The number of strands found in such domains may differ from one protein to another. β-sandwich domains are subdivided in a variety of different folds. The immunoglobulin-type fold found in antibodies (Ig-fold) consists of a sandwich arrangement of 7-9 antiparallel β-strands arranged in two β-sheets with a Greek-key topology. The Greek-key topology is also found in Human Transthyretin. The jelly-roll topology is found in carbohydrate binding proteins such as concanavalin A and various lectins, in the collagen binding domain of ''Staphylococcus aureus'' Adhesin and in modules that bind fibronectin as found in Tenascin Tenascins are extracellular matrix glycoproteins. They are abundant in the extracellular matrix of developing vertebrate embryos and they reappear around healing wounds and in the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Structure
Protein secondary structure is the local spatial conformation of the polypeptide backbone excluding the side chains. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary structure elements typically spontaneously form as an intermediate before the protein protein folding, folds into its three dimensional protein tertiary structure, tertiary structure. Secondary structure is formally defined by the pattern of hydrogen bonds between the Amine, amino hydrogen and carboxyl oxygen atoms in the peptide backbone chain, backbone. Secondary structure may alternatively be defined based on the regular pattern of backbone Dihedral angle#Dihedral angles of proteins, dihedral angles in a particular region of the Ramachandran plot regardless of whether it has the correct hydrogen bonds. The concept of secondary structure was first introduced by Kaj Ulrik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit the air, soil, water, Hot spring, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria play a vital role in many stages of the nutrient cycle by recycling nutrients and the nitrogen fixation, fixation of nitrogen from the Earth's atmosphere, atmosphere. The nutrient cycle includes the decomposition of cadaver, dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Tryptophan is named after the digestive enzymes trypsin, which were used in its first isolation from casein proteins. It was assigned the one-letter symbol W based on the double ring being visually suggestive to the bulky letter. Function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |