|
Calcination
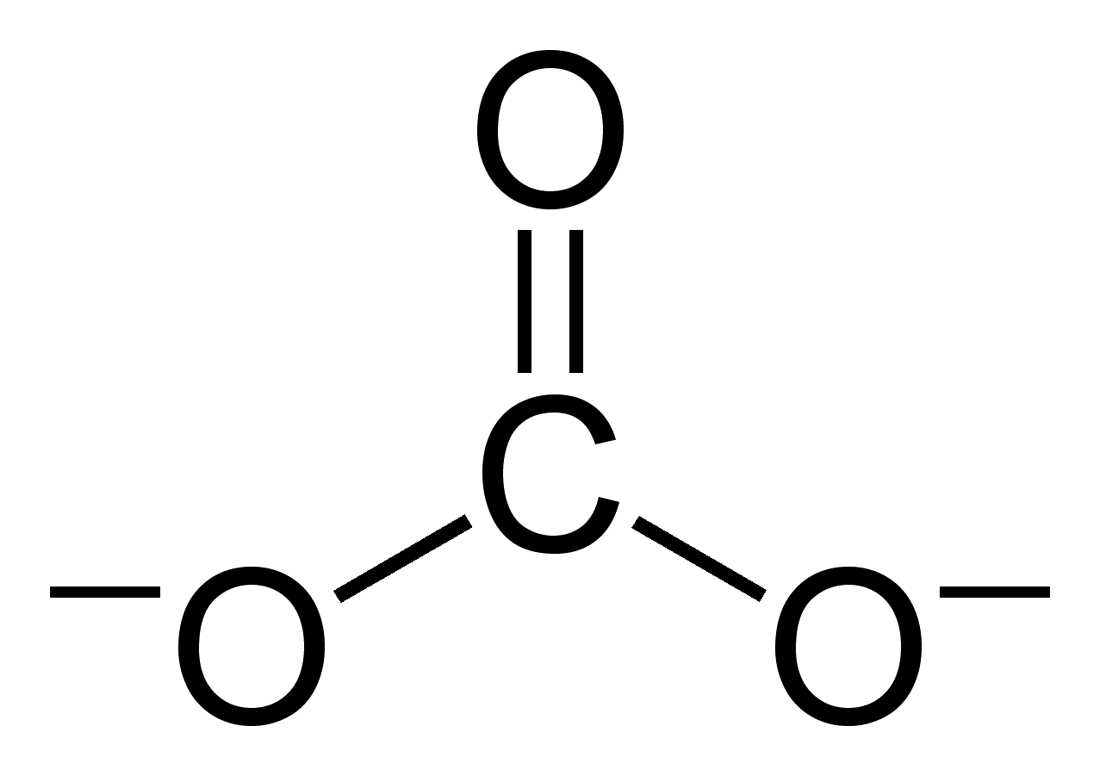
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (). A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled atmosphere. Etymology The process of calcination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petroleum Coke
Petroleum coke, abbreviated coke, pet coke or petcoke, is a final carbon-rich solid material that derives from oil refinery, oil refining, and is one type of the group of fuels referred to as Coke (fuel), cokes. Petcoke is the coke that, in particular, derives from a final cracking (chemistry), cracking process—a thermo-based chemical engineering process that splits long chain hydrocarbons of petroleum into shorter chains—that takes place in units termed coker units. (Other types of Coke (fuel), coke are derived from coal.) Stated succinctly, coke is the "carbonization product of high-boiling hydrocarbon fractions obtained in petroleum processing (heavy residues)". Petcoke is also produced in the production of synthetic crude oil (syncrude) from bitumen extracted from Canada's oil sands and from Venezuela's Orinoco oil sands. In petroleum coker units, residual oils from other distillation processes used in petroleum refining are treated at a high temperature and pressure leavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kiln
A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or Chemical Changes, chemical changes. Kilns have been used for millennia to turn objects made from clay into pottery, Tile, tiles and bricks. Various industries use rotary kilns for pyroprocessing (to calcinate ores, such as limestone to Lime (material), lime for Cement kiln, cement) and to transform many other materials. Etymology According to the Oxford English Dictionary, kiln was derived from the words cyline, cylene, cyln(e) in Old English, in turn derived from Latin ''culina'' ('kitchen'). In Middle English, the word is attested as kulne, kyllne, kilne, kiln, kylle, kyll, kil, kill, keele, kiele. In Greek the word ''καίειν, kaiein'', means 'to burn'. Pronunciation The word 'kiln' was originally pronounced 'kil' with the 'n' silent, as is referenced in ''Webster's Dictionary of 1828'' and in ''English Words as Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide (of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Treatment
Thermal treatment is any list of solid waste treatment technologies, waste treatment technology that involves high temperatures in the processing of the waste Raw material, feedstock. Commonly this involves the combustion of waste materials. Systems that are generally considered to be thermal treatment include: *Cement kiln *Gasification *Incineration *Mechanical heat treatment *Pyrolysis *Thermal depolymerization *Waste autoclaves See also *Anaerobic digestion *List of solid waste treatment technologies *Mechanical biological treatment *Waste-to-energy *Pyrolysis References {{Waste Thermal treatment, Waste management Waste treatment technology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also Crystallization, crystallizes as translucent crystals of selenite (mineral), selenite. It forms as an evaporite mineral and as a Mineral hydration, hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on Scratch hardness, scratch hardness comparison. Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Etymology and history The word ''wikt:gypsum, gypsum'' is derived from the Greek language, Greek word (), "plaster". Because the quarry, quarries of the Montmartre district of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Ore
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, , the chief constituent of limestone (as well as the main component of mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Of Crystallization
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a chemical substance, substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the Crystal structure, crystalline framework of a metal complex or a salt (chemistry), salt, which is not directly chemical bond, bonded to the metal cation. Upon crystallization from water, or water-containing solvents, many chemical compound, compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to Inorganic compound, inorganic salts, proteins crystallize with large amounts of water i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite. Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum. Rutile derives its name from the Latin ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality. Occurrence Rutile is a comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anatase
Anatase is a metastable mineral form of titanium dioxide (TiO2) with a Tetragonal crystal system, tetragonal crystal structure. Although colorless or white when pure, anatase in nature is usually a black solid due to impurities. Three other Polymorphism (materials science), polymorphs (or mineral forms) of titanium dioxide are known to occur naturally: brookite, akaogiite, and rutile, with rutile being the most common and most Chemical stability, stable of the bunch. Anatase is formed at relatively low temperatures and found in minor concentrations in Igneous rock, igneous and Metamorphic rock, metamorphic rocks. Glass coated with a thin film of TiO2 shows Anti-fog, antifogging and Self-cleaning surfaces, self-cleaning properties under ultraviolet radiation. Anatase is always found as small, isolated, and sharply developed crystals, and like rutile, it crystallizes in a Tetragonal crystal system, tetragonal system. Anatase is metastable at all temperatures and pressures, with ru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluidized Bed Reactor
A fluidized bed reactor (FBR) is a type of reactor device that can be used to carry out a variety of multiphase chemical reactions. In this type of reactor, a fluid (gas or liquid) is passed through a solid granular material (usually a catalyst) at high enough speeds to suspend the solid and cause it to behave as though it were a fluid. This process, known as fluidization, imparts many important advantages to an FBR. As a result, FBRs are used for many industrial applications. Basic principles The solid substrate material (the catalytic material upon which chemical species react) in the fluidized bed reactor is typically supported by a porous plate, known as a distributor.Howard, J. R. (1989). ''Fluidized Bed Technology: Principles and Applications.'' New York, NY: Adam Higler. The fluid is then forced through the distributor up through the solid material. At lower fluid velocities, the solids remain in place as the fluid passes through the voids in the material. This is kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Devitrification
Devitrification is the process of crystallization in a formerly crystal-free (amorphous) glass. The term is derived from the Latin ''vitreus'', meaning '' glassy'' and '' transparent''. Devitrification in glass art Devitrification occurs in glass art during the firing process of fused glass whereby the surface of the glass develops a whitish scum, crazing, or wrinkles instead of a smooth glossy shine, as the molecules in the glass change their structure into that of crystalline solids. While this condition is normally undesired, in glass art it is possible to use devitrification as a deliberate artistic technique. Causes of devitrification, commonly referred to as "devit", can include holding a high temperature for too long, which causes the nucleation of crystals. The presence of foreign residue such as dust on the surface of the glass or inside the kiln prior to firing can provide nucleation points where crystals can propagate easily. The chemical compositions of some types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |