|
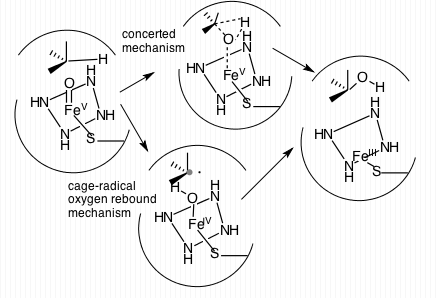
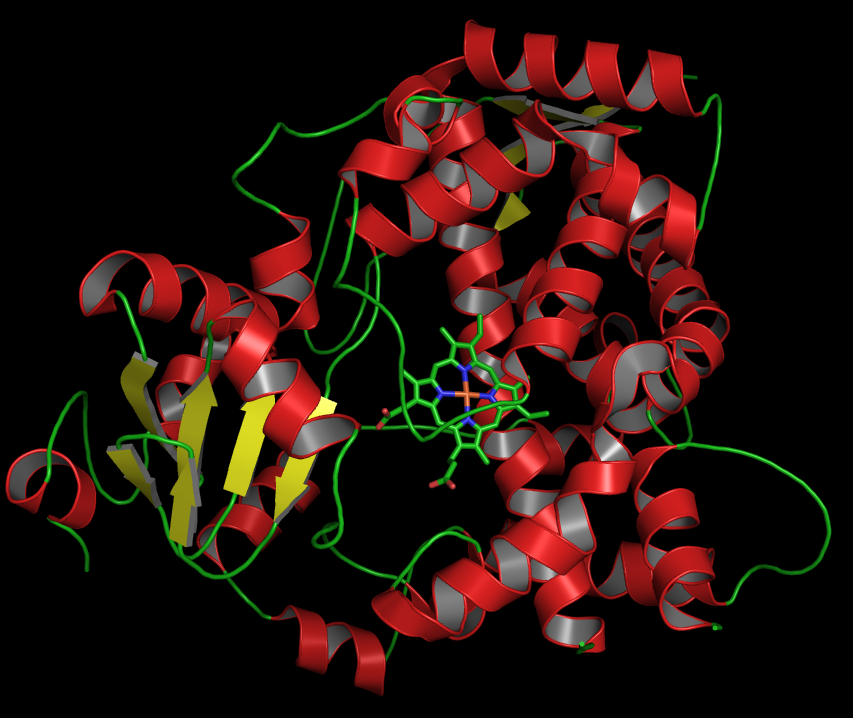
CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by ''CYP3A4'' gene. It organic redox reaction, oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs that are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will, therefore, either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A5
Cytochrome P450 3A5 is a protein that in humans is encoded by the ''CYP3A5'' gene. Tissue distribution ''CYP3A5'' encodes a member of the cytochrome P450 superfamily of enzymes. Like most of the cytochrome P450, the CYP3A5 is expressed in the prostate and the liver. It is also expressed in epithelium of the small intestine and large intestine for uptake and in small amounts in the bile duct, nasal mucosa, kidney, adrenal cortex, epithelium of the gastric mucosa with intestinal metaplasia, gallbladder, intercalated ducts of the pancreas, chief cells of the parathyroid and the corpus luteum of the ovary (at protein level). Clinical significance The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum and its expression is induced by glucocorticoids and some pharmacological agents. The enzyme metabolizes drugs such as nif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grapefruit
The grapefruit (''Citrus'' × ''paradisi'') is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The flesh of the fruit is segmented and varies in color from pale yellow to dark red. Grapefruits originated in Barbados in the 18th century. They are a citrus hybrid that was created through an accidental cross between the sweet orange (''C.'' × ''sinensis'') and the pomelo (''C. maxima''), both of which were introduced to the Caribbean from Asia in the 17th century. It has also been called the 'forbidden fruit'. In the past it was called the ''pomelo'', but that term is now mostly used as the common name for ''Citrus maxima''. Grapefruit–drug interactions are common, as the juice contains furanocoumarins that interfere with the metabolism of many drugs. This can prolong and intensify the effects of those drugs, leading to multiple side-effects such as abnormal heart rhythms, bleeding inside the stomach, low blood pressure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythromycin
Erythromycin is an antibiotic used for the treatment of a number of bacterial infections. This includes respiratory tract infections, skin infections, chlamydia infections, pelvic inflammatory disease, and syphilis. It may also be used during pregnancy to prevent Group B streptococcal infection in the newborn, and to improve delayed stomach emptying. It can be given intravenously and by mouth. An eye ointment is routinely recommended after delivery to prevent eye infections in the newborn. Common side effects include abdominal cramps, vomiting, and diarrhea. More serious side effects may include ''Clostridioides difficile'' colitis, liver problems, prolonged QT, and allergic reactions. It is generally safe in those who are allergic to penicillin. Erythromycin also appears to be safe to use during pregnancy. While generally regarded as safe during breastfeeding, its use by the mother during the first two weeks of life may increase the risk of pyloric stenosis in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ciclosporin
Ciclosporin, also spelled cyclosporine and cyclosporin, is a calcineurin inhibitor, used as an immunosuppressant medication. It is taken Oral administration, orally or intravenously for rheumatoid arthritis, psoriasis, Crohn's disease, nephrotic syndrome, eczema, and in organ transplants to prevent transplant rejection, rejection. It is also used as eye drops for keratoconjunctivitis sicca (dry eyes). Common side effects include high blood pressure, headache, kidney problems, increased hair growth, and vomiting. Other severe side effects include an increased risk of infection, liver problems, and an increased risk of lymphoma. Blood levels of the medication should be checked to decrease the risk of side effects. Use during pregnancy may result in preterm birth; however, ciclosporin does not appear to cause birth defects. Ciclosporin is believed to work by decreasing the function of lymphocytes. It does this by forming a complex with cyclophilin to block the phosphatase act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazepam
Diazepam, sold under the brand name Valium among others, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is used to treat a range of conditions, including anxiety disorder, anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause amnesia, memory loss during certain medical procedures. It can be taken Oral administration, orally (by mouth), as a suppository inserted into the rectum, Intramuscular injection, intramuscularly (injected into muscle), Intravenous therapy, intravenously (injection into a vein) or used as a nasal spray. When injected intravenously, effects begin in one to five minutes and last up to an hour. When taken by mouth, effects begin after 15 to 60 minutes. Common side effects include sleepiness and trouble with coordination. Serious side effects are rare. They include increased risk of suicide, decreased breathing, and a paradoxical increased risk of seizures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxygenase
Epoxygenases are a set of membrane-bound, heme-containing cytochrome P450 (CYP450 or just CYP) enzymes that metabolize polyunsaturated fatty acids (PUFAs) to epoxide products that have a range of biological activities. The most thoroughly-studied substrate of the CYP epoxygenases is the PUFA arachidonic acid (AA). Eicosanoids are created from AA in three pathways: # Cyclooxygenases metabolize AA to various prostaglandin, thromboxane, and prostacyclin metabolites. # Lipoxygenases metabolize AA to hydroxyeicosatetraenoic acids (e.g. 5-HETE or 12-HETE) and leukotrienes (e.g. leukotriene B4 or leukotriene C4). # CYP epoxygenases metabolize AA to epoxyeicosatrienoic acids (EETs). Like the first two pathways, the third acts as a signaling pathway wherein the eicosatrienoic acid epoxide products work as secondary signals to activate their parent or nearby cells and thereby orchestrate functional responses. However, these enzymes are not limited to metabolizing AA to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicated
Toxication, toxification or toxicity exaltation is the conversion of a chemical compound into a more toxic form in living organisms or in substrates such as soil or water. The conversion can be caused by enzyme, enzymatic metabolism in the organisms, as well as by abiotic chemical reactions. While the parent drug is usually less active, both the parent drug and its metabolite can be chemically active and cause toxicity, leading to mutagenesis, teratogenesis, and carcinogenesis. Different classes of enzymes, such as P450 monooxygenases, epoxide hydrolase, or acetyltransferases can catalyze the process in the cell, mostly in the liver. Parent non-toxic chemicals are generally referred to as ''protoxins''. While toxication is generally undesirable, in certain cases it is required for the ''in vivo'' conversion of a prodrug to a metabolite with desired pharmacological or toxicological activity. Codeine is an example of a prodrug, metabolized in the body to the active compounds morphine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Codeine
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, ''Papaver somniferum''. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of addiction and overdose. Common side effects include nausea, vomiting, constipation, itchiness, lightheadedness, and drowsiness. Serious ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
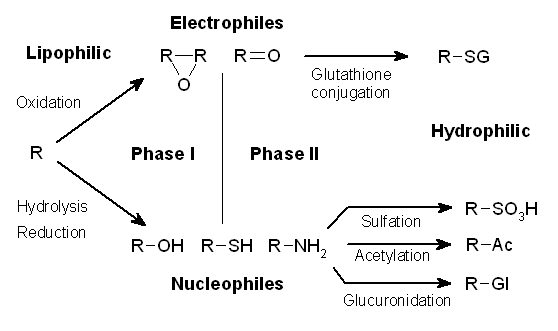
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics. Metabolism is one of the stages (see ADME) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body. The metabolism of pharmaceutical drugs is an important as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroquine
Chloroquine is an antiparasitic medication that treats malaria. It works by increasing the levels of heme in the blood, a substance toxic to the malarial parasite. This kills the parasite and stops the infection from spreading. Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication. Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus. While it has not been formally studied in pregnancy, it appears safe. It is taken by mouth. It was studied to treat COVID-19 early in the COVID-19 pandemic, pandemic, but these studies were largely halted in the northern summer of 2020, and the National Institutes of Health, NIH does not recommend its use for this purpose. Common side effects include muscle problems, loss of appetite, diarrhea, and skin rash. Serious side effects include problems with vision, muscle damage, seizures, and aplas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
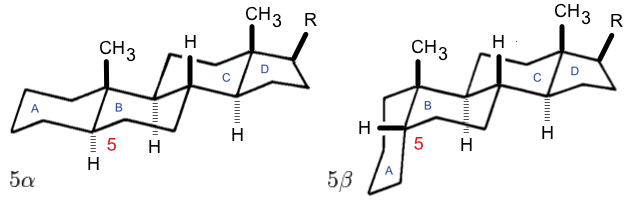
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furanocoumarin
The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. Most of the plant species found to contain furanocoumarins belong to a handful of plant families. The families Apiaceae and Rutaceae (citrus family) include the largest numbers of plant species that contain furanocoumarins. The families Moraceae and Fabaceae include a few widely distributed plant species that contain furanocoumarins. Generally, furanocoumarins are most abundant in plants that have flowered and in ripe seeds and fruits. (An exception is the common fig where furanocoumarins are found chiefly in the milky sap of the leaves and shoots but not the fruits. Cited in McGovern and Barkley 2000, section&nbsPhytophotodermatitis) During the early stages of plant growth, their presence is not easily detected. Structure The chemical structure of furanocoumarins consists of a furan ring fused with a coumarin. The furan ring may be fused in various ways, producin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |