|
CYB5A
Cytochrome b5, form A (gene name CYB5A), is a human microsomal cytochrome b5. Cytochrome b5 is a membrane bound hemoprotein which functions as an electron carrier for several membrane bound oxygenases. It has two isoforms produced by alternative splicing. Isoform 1 is bound to the cytoplasmic side of the endoplasmic reticulum. It has a C-terminal transmembrane alpha-helix. Isoform 2 was found in cytoplasm. Defects in CYB5A are the cause of type IV hereditary methemoglobinemia Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications ma .... References Further reading * * * * * * * * * * * * * * * * * * * * Transmembrane proteins {{membrane-protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome B5
Cytochromes ''b''5 are ubiquitous electron transport hemoproteins found in animals, plants, fungi and purple bacteria, purple phototrophic bacteria. The microsome, microsomal and mitochondrial variants are membrane-bound, while bacterial and those from erythrocytes and other Biological tissue#Animal tissues, animal tissues are water-soluble. The family of cytochrome ''b''5-like proteins includes (besides cytochrome ''b''5 itself) hemoprotein domains covalently associated with other redox domains in flavocytochrome cytochrome ''b''2 (L-lactate dehydrogenase; ), sulfite oxidase (), plant and fungal nitrate reductases (, , ), and plant and fungal cytochrome ''b''5/acyl lipid desaturase fusion proteins. Structure 3-D structures of a number of cytochrome ''b''5 and yeast flavocytochrome ''b''2 are known. The fold belongs to the α+β class, with two hydrophobic cores on each side of a β-sheet. The larger hydrophobic core constitutes the heme-binding pocket, closed off on each side ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microsomal
In cell biology, microsomes are heterogeneous vesicle-like artifacts (~20-200 nm diameter) re-formed from pieces of the endoplasmic reticulum (ER) when eukaryotic cells are broken-up in the laboratory; microsomes are not present in healthy, living cells. Rough (containing ribosomes) and smooth (without ribosomes) microsomes are made from the endoplasmic reticulum through cell disruption. These microsomes have an inside that is exactly the same as the endoplasmic reticulum lumen. Both forms of microsomes can be purified by a process known as equilibrium density centrifugation. Rough and smooth microsomes do differ in their proteins and rough microsomes have shown occurrence of translation and translocation at the same time besides certain exceptions from proteins in yeast. Signal Hypothesis The Signal Hypothesis was postulated by Günter Blobel and David Sabatini in 1971, stating that a unique peptide sequence is encoded by mRNA specific for proteins destined for translocat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoprotein
A hemeprotein (or haemprotein; also hemoprotein or haemoprotein), or heme protein, is a protein that contains a heme prosthetic group. They are a very large class of metalloproteins. The heme group confers functionality, which can include oxygen carrying, oxygen reduction, electron transfer, and other processes. Heme is bound to the protein either covalently or noncovalently or both. The heme consists of iron cation bound at the center of the conjugate base of the porphyrin, as well as other ligands attached to the "axial sites" of the iron. The porphyrin ring is a planar dianionic, tetradentate ligand. The iron is typically Fe2+ or Fe3+. One or two ligands are attached at the axial sites. The porphyrin ring has four nitrogen atoms that bind to the iron, leaving two other coordination positions of the iron available for bonding to the histidine of the protein and a divalent atom. Hemeproteins probably evolved to incorporate the iron atom contained within the protoporphyrin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygenase
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14. Structure Most oxygenases contain either a metal, usually iron, or an organic cofactor, usually flavin. These cofactors interact with O2, leading to its transfer to substrate. Oxygenases constitute a major intracellular source of iron and carbon monoxide Mechanism Two types of oxygenases are recognized: *Monooxygenases, or mixed function oxidase, transfer one oxygen atom to the substrate, and reduce the other oxygen atom to water. *Dioxygenases, or oxygen transferases, incorporate both atoms of molecular oxygen (O2) into the product(s) of the reaction. Among the most common monooxygenases are the cytochrome P450 oxidase Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments (exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions of genes revealed by the human genome project and the large diversity of proteins seen in an organism: different proteins enc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
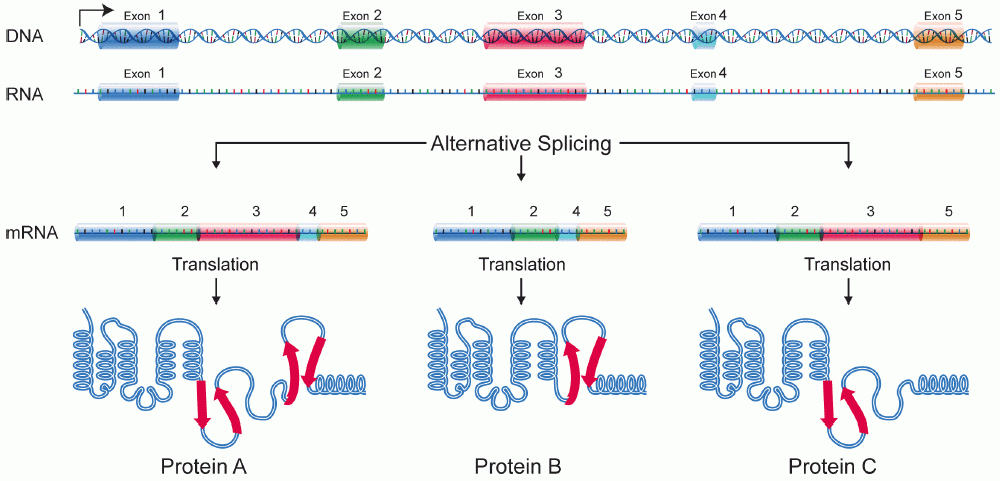
Alternative Splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative RNA splicing, splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene may be included within or excluded from the final RNA product of the gene. This means the exons are joined in different combinations, leading to different splice variants. In the case of protein-coding genes, the proteins translated from these splice variants may contain differences in their amino acid sequence and in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most of the observed splice variants ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methemoglobinemia
Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications may include seizures and heart arrhythmias. Methemoglobinemia can be due to certain medications, chemicals, or food or it can be inherited. Substances involved may include benzocaine, nitrites, or dapsone. The underlying mechanism involves some of the iron in hemoglobin being converted from the ferrous [Fe2+] to the ferric [Fe3+] form. The diagnosis is often suspected based on symptoms and a Hypoxia (medical), low blood oxygen that does not improve with oxygen therapy. Diagnosis is confirmed by a blood gas. Treatment is generally with oxygen therapy and methylene blue. Other treatments may include vitamin C, exchange transfusion, and hyperbaric oxygen therapy. Outcomes are generally good with treatment. Methemoglobinemia is relatively unc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |