|
CHAPS Detergent
CHAPS is a zwitterionic surfactant used in the laboratory to solubilize biological macromolecules such as proteins. It may be synthesized from cholic acid and is zwitterionic due to its quaternary ammonium and sulfonate groups; it is structurally similar to certain bile acids, such as taurodeoxycholic acid and taurochenodeoxycholic acid. It is used as a non- denaturing detergent in the process of protein purification and is especially useful in purifying membrane proteins, which are often sparingly soluble or insoluble in aqueous solution due to their native hydrophobicity. CHAPS is an abbreviation for 3- 3-cholamidopropyl)dimethylammonio1-propanesulfonate. A related detergent, called CHAPSO, has the same basic chemical structure with an additional hydroxyl functional group; its full chemical name is 3- 3-cholamidopropyl)dimethylammonio2-hydroxy-1-propanesulfonate. Both detergents have low light absorbance in the ultraviolet region of the electromagnetic spectrum, which is usefu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups. : (1,2- dipolar compounds, such as ylides, are sometimes excluded from the definition.) Some zwitterions, such as amino acid zwitterions, are in chemical equilibrium with an uncharged "parent" molecule. Betaines are zwitterions that cannot isomerize to an all-neutral form, such as when the positive charge is located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot isomerize. Amino acids Tautomerism of amino acids follows this stoichiometry: : The ratio of the concentrations of the two species in solution is independent of pH. It has been suggested, on the basis of theoretical analysis, that the zwitterion is stabilized in aqueous solution by hydrogen bonding with solvent water molecules. Analysis of neutron diffraction data for g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
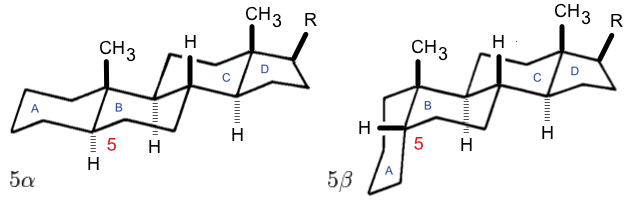
Steroids
A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in fungi, plants, and animals. All steroids are manufactured in cells from a sterol: cholesterol (animals), lanosterol ( opisthokonts), or cycloartenol (plants). All three of these molecules are produced via cyclization of the triterpene squalene. Structure The steroid nucleus ( core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in four fused rings: three six-member cyclohexane rings (rings A, B and C in the first illus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taurochenodeoxycholic Acid
Taurochenodeoxycholic acid is a bile acid formed in the liver of most species, including humans, by conjugation of chenodeoxycholic acid with taurine. It is secreted into bile and then into the intestine. It is usually ionized at physiologic pH. However, although it can be crystallized as the sodium salt. It acts as a detergent to solubilize fats in the small intestine and is itself absorbed by active transport in the terminal ileum. It is used as a cholagogue and choleretic. Substantial evidence indicates that high circulating bile acids promote colon cancer risk. In a prospective study, positive associations were observed between prediagnostic plasma levels of seven conjugated bile acid metabolites, including taurochenodeoxycholic acid, and colon cancer risk. See also * Tauroursodeoxycholic acid, an epimer * See article about Taurodeoxycholic acid as an interferent in Perfluorooctanesulfonic acid Perfluorooctanesulfonic acid (PFOS) (conjugate acid, conjugate base perfluor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taurodeoxycholic Acid
Taurodeoxycholic acid is a bile acid. This compound is a closely related isomer of taurochenodeoxycholic acid and tauroursodeoxycholic acid sharing the exact molecular formula and molecular weight. Taurodeoxycholic acid and its isomers have molecular masses similar to perfluorooctanesulfonic acid (PFOS) and therefore may interfere with interpretation of mass spectrometry data, leading to a false indication of the presence of PFOS in a biological sample. Serum concentration of taurodeoxycholic acid, a downstream microbial metabolite of cholic acid, is associated with a strong increased risk of colorectal cancer among women. Also, the determination of taurodeoxycholic acid 3-sulfate in blood samples may potentially be useful as a risk factor and screening biomarker for lung cancer Lung cancer, also known as lung carcinoma, is a malignant tumor that begins in the lung. Lung cancer is caused by genetic damage to the DNA of cells in the airways, often caused by cigarett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high frequency these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. Radio waves, at the low-frequency end of the spectrum, have the lowest photon energy and the longest wavelengths—thousands of kilometers, or more. They can be emitted and received by antenna (radio), antennas, and pass through the atmosphere, foliage, and most building materials. Gamma rays, at the high-frequency end of the spectrum, have the highest photon energies and the shortest wavelengths—much smaller than an atomic nucleus. Gamma rays, X-rays, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |