|
Butane
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at room temperature and pressure. Butanes are a trace components of natural gases (NG gases). The other hydrocarbons in NG include propane, ethane, and especially methane, which are more abundant. Liquefied petroleum gas is a mixture of propane and some butanes. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane (for organic compounds). History The first synthesis of butane was accidentally achieved by British chemist Edward Frankland in 1849 from ethyl iodide and zinc, but he had not realized that the ethyl radical dimerized and misidentified the substance. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butane Simple
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefy, liquefied gases that quickly vaporize at room temperature and pressure. Butanes are a trace components of natural gases (NG gases). The other hydrocarbons in NG include propane, ethane, and especially methane, which are more abundant. Liquefied petroleum gas is a mixture of propane and some butanes. The name butane comes from the root IUPAC nomenclature of organic chemistry#Alkanes, but- (from butyric acid, named after the Greek word for butter) and the suffix alkanes, -ane (for organic compounds). History The first synthesis of butane was accidentally achieved by British chemist Edward Frankland in 1849 from ethyl iodide and zinc, but he had not realized that the ethyl radical dimerized and misidentified the substance. It was discovered in crude petroleum in 1864 by Edm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane. Production Isobutane is obtained by isomerization of butane. Uses Isobutane is the principal feedstock in alkylation units of refineries. Using isobutane, gasoline-grade "blendstocks" are generated with high branching for good combustion characteristics. Typical products created with isobutane are 2,4-dimethylpentane and especially 2,2,4-trimethylpentane. Solvent In the Chevron Phillips slurry process for making high-density polyethylene, isobutane is used as a diluent. As the slurried polyethylene is removed, isobutane is "flashed" off, and condensed, and recycled back into the loop reactor for this purpose. Prec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like hexacontane () or 4-methyl-5-(1-methylethyl) octane, an isomer of dodecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or polycycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum refining, it is often a constituent of liquefied petroleum gas (LPG), which is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation; other constituents of LPG may include propene, propylene, butane, butene, butylene, butadiene, and isobutylene. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane has lower volumetric energy density than gasoline or coal, but has higher gravimetric energy density than them and burns more cleanly. Propane gas has become a popular choice for barbecues and portable stoves because its low −42 °C boiling point makes it vaporise inside pressurised liquid containers (it exists in two pha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorobutane
Perfluorobutane (PFB) is an inert, high-density colorless gas. It is a simple fluorocarbon with a ''n''-butane skeleton and all the hydrogen atoms replaced with fluorine atoms. Uses Perfluorobutane can replace Halon 1301 in fire extinguishers, as well as the gas component for newer generation microbubble ultrasound contrast agents. Sonazoid is one such microbubble formulation developed by Amersham Health that uses perfluorobutane for the gas core. Due to its inert nature and high density, inhaling perfluorobutane makes one's voice deeper. Caution is advised as heavier gases are difficult to breathe out and may cause inert gas asphyxiation. Environmental impacts If perfluorobutane is released to the environment, it will not be broken down in air. It is not expected to be broken down by sunlight. It will move into air from soil and water surfaces. If it is exposed to conditions of extreme heat from misuse, equipment failure, etc., toxic decomposition products including hydroge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquefied Petroleum Gas
Liquefied petroleum gas, also referred to as liquid petroleum gas (LPG or LP gas), is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, Butane, ''n''-butane and isobutane. It can also contain some propylene, butylene, and isobutylene/Isobutylene, isobutene. LPG is used as a fuel gas in HVAC, heating appliances, cooking equipment, and vehicles, and is used as an aerosol propellant and a refrigerant, replacing chlorofluorocarbons in an effort to reduce the damage it causes to the ozone layer. When specifically used as a vehicle fuel, it is often referred to as autogas or just as Autogas#Terminology variations and confusion, gas. Varieties of LPG that are bought and sold include mixes that are mostly propane (), mostly butane (), and, most commonly, mixes including both propane and butane. In the northern hemisphere winter, the mixes contain more propane, while in summer, they contain more butane. In the United States, mainly two grad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
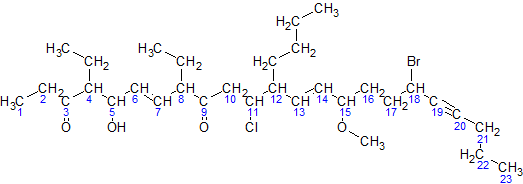
IUPAC Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of Organic Chemistry'' (informally called thBlue Book. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |