|
Binary Compounds
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended solids. Famous examples zinc sulfide, which contains zinc and sulfur, and tungsten carbide, which contains tungsten and carbon. Phases with higher degrees of complexity feature more elements, e.g. three elements in ternary phase In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements. While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases re ...s, four elements in quaternary phases. These phases exhibit a higher degree of complexity due to the interaction of these elements at different conditions. References Chemical compounds {{chem-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
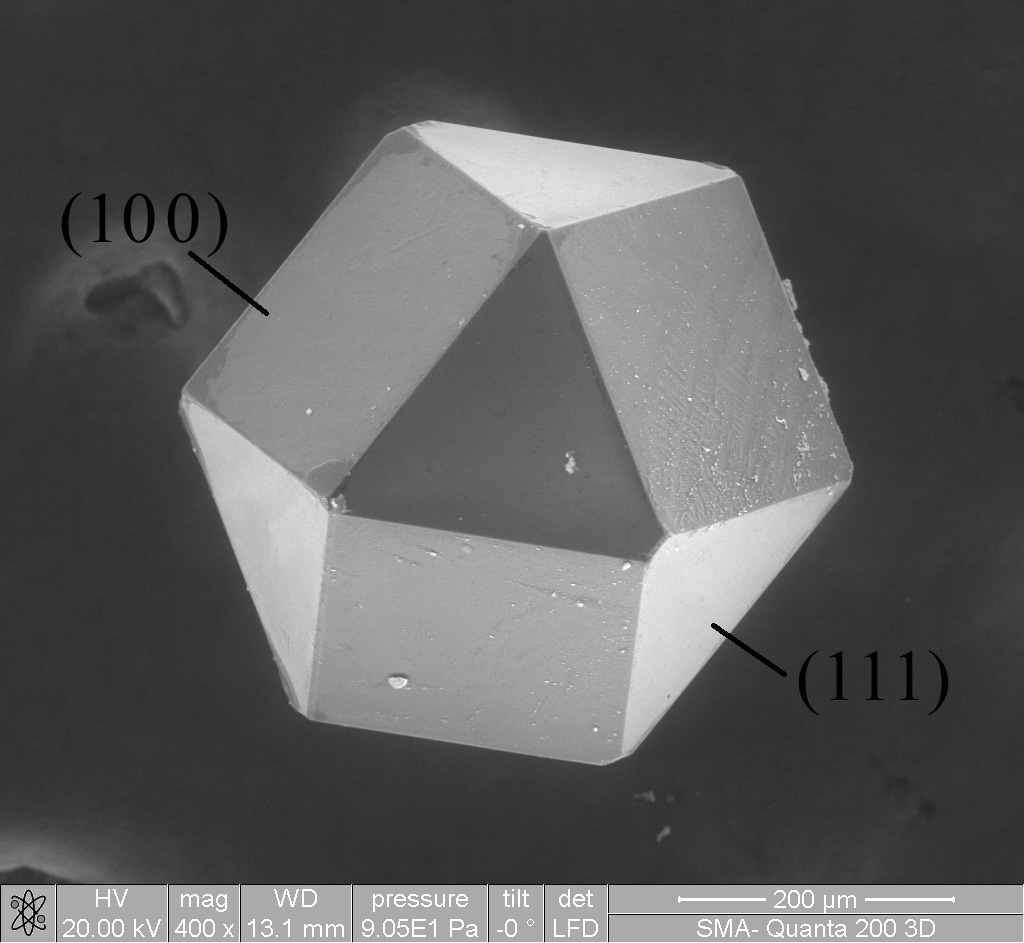
NaCl Polyhedra
Sodium chloride , commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather. Uses In addition to the many familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.Westphal, Gisbert ''et al.'' (2002) "Sodium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim . Chemical functions Salt is used, directly or indirectly, in the prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Materials Chemistry
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries. The intellectual origins of materials science stem from the Age of Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy. Materials science still incorporates elements of physics, chemistry, and engineering. As such, the field was long considered by academic institutions as a sub-field of these related fields. Beginning in the 1940s, materials science began to be more widely recognized as a specific and distinct field of science and engineering, and major technical universities around the world created dedicated schools for its study. Materials scientists emphasize understanding how the history of a material (''processing'') influences its structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, an anthelmintic and a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. methane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
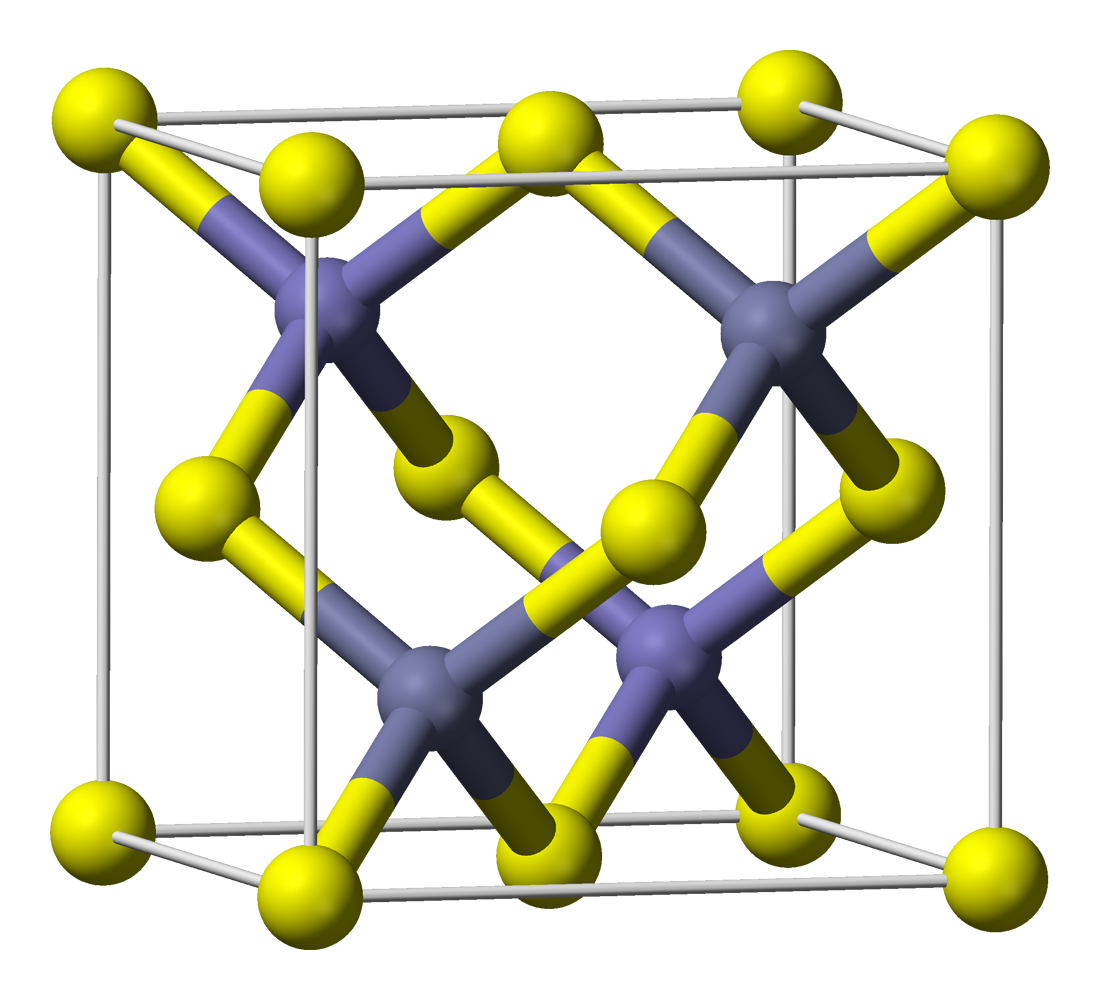
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparency and translucency, transparent, and it is used as a window for visible light, visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism (materials science), polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. Applications Luminescent mate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Carbide
Tungsten carbide (chemical formula: ) is a carbide containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed into shapes through sintering for use in industrial machinery, engineering facilities, molding blocks, cutting tools, chisels, abrasives, armor-piercing bullets and jewelry. Tungsten carbide is approximately three times as stiff as steel, with a Young's modulus of approximately 530–700 GPa, and is twice as dense as steel. It is comparable with corundum (α- ) in hardness, approaching that of a diamond, and can be polished and finished only with abrasives of superior hardness such as cubic boron nitride and diamond. Tungsten carbide tools can be operated at cutting speeds much higher than high-speed steel (a special steel blend for cutting tools). Tungsten carbide powder was first synthesized by H. Moissan in 1893, and the industrial production of the cemented form starte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
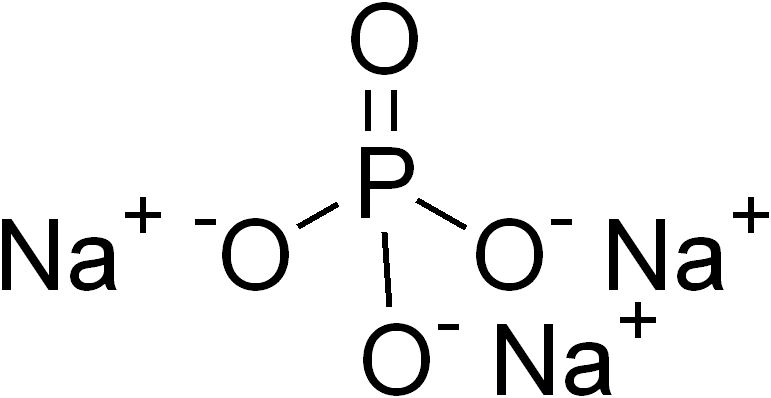
Ternary Phase
In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements. While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases refer to extended solids. The perovskites are a famous example. Binary phases, with only two elements, have lower degrees of complexity than ternary phases. With four elements, quaternary phases are more complex. The number of isomers of a ternary compound provide a distinction between inorganic and organic chemistry: "In inorganic chemistry one or, at most, only a few compounds composed of any two or three elements were known, whereas in organic chemistry the situation was very different." Ternary crystalline compounds An example is sodium phosphate, . The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3–. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of a te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Phase
In materials chemistry, a quaternary phase is a chemical compound containing four elements. Some compounds can be molecular or ionic, examples being chlorodifluoromethane () and sodium bicarbonate (). More typically quaternary phase refers to extended solids. A famous example are the yttrium barium copper oxide superconductors. See also *Binary compound *Ternary compound In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements. While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases r ... References Chemical compounds {{chem-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compounds
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |