|
1913 In Science
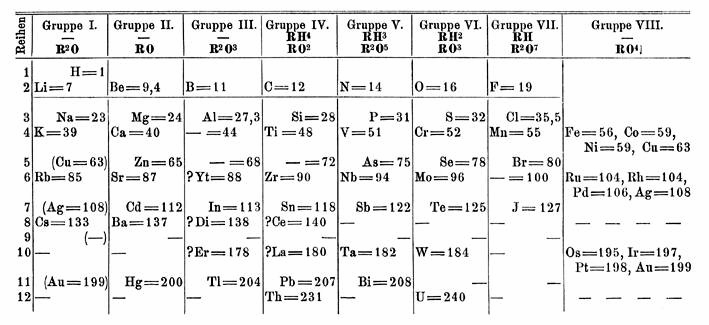
The year 1913 in science and technology involved some significant events, listed below. Astronomy * February 9 – Meteor procession of February 9, 1913 visible along a great circle arc 6, across the Americas. Astronomer Clarence Chant concludes that the source was a small, short-lived natural satellite of the Earth. * Berlin Observatory moves to Babelsberg. Biology * William Temple Hornaday publishes Our Vanishing Wild Life: Its Extermination and Preservation'. Chemistry * February – Daniel J. O'Conor and Herbert A. Faber file for a United States patent on the Composite material, composite plastic laminate Formica (plastic), Formica. * Elmer McCollum and Marguerite Davis at the University of Wisconsin–Madison, and Lafayette Mendel and Thomas Burr Osborne (chemist), Thomas Burr Osborne at Yale University independently discover Vitamin A. * Protactinium is first identified by Oswald Helmuth Göhring and Kasimir Fajans. * Henry Moseley shows that nuclear charge is the real ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Wisconsin–Madison
The University of Wisconsin–Madison (University of Wisconsin, Wisconsin, UW, UW–Madison, or simply Madison) is a public land-grant research university in Madison, Wisconsin, United States. It was founded in 1848 when Wisconsin achieved statehood and is the flagship campus of the University of Wisconsin System. The main campus is located on the shores of Lake Mendota; the university also owns and operates a arboretum south of the main campus. UW–Madison is organized into 13 schools and colleges, which enrolled approximately 34,200 undergraduate and 14,300 graduate and professional students in 2024. Its academic programs include 136 undergraduate majors, 148 master's degree programs, and 120 doctoral programs. Wisconsin is one of the founding members of the Association of American Universities. It is considered a Public Ivy and is classified as an R1 University. UW–Madison was also the home of both the prominent "Wisconsin School" of economics and diplomatic h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high frequency these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. Radio waves, at the low-frequency end of the spectrum, have the lowest photon energy and the longest wavelengths—thousands of kilometers, or more. They can be emitted and received by antenna (radio), antennas, and pass through the atmosphere, foliage, and most building materials. Gamma rays, at the high-frequency end of the spectrum, have the highest photon energies and the shortest wavelengths—much smaller than an atomic nucleus. Gamma rays, X-rays, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ranging from 10 Nanometre, nanometers to 10 Picometre, picometers, corresponding to frequency, frequencies in the range of 30 Hertz, petahertz to 30 Hertz, exahertz ( to ) and photon energies in the range of 100 electronvolt, eV to 100 keV, respectively. X-rays were discovered in 1895 in science, 1895 by the German scientist Wilhelm Röntgen, Wilhelm Conrad Röntgen, who named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medical diagnostics (e.g., checking for Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom which contains protons, neutrons and electrons, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in daltons (making a quantity called the " relative isotopic mass"), is within 1% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats. In other words, it is the distance between consecutive corresponding points of the same ''phase (waves), phase'' on the wave, such as two adjacent crests, troughs, or zero crossings. Wavelength is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The multiplicative inverse, inverse of the wavelength is called the ''spatial frequency''. Wavelength is commonly designated by the Greek letter lambda (''λ''). For a modulated wave, ''wavelength'' may refer to the carrier wavelength of the signal. The term ''wavelength'' may also apply to the repeating envelope (mathematics), envelope of modulated waves or waves formed by Interference (wave propagation), interference of several sinusoids. Assuming a sinusoidal wave moving at a fixed phase velocity, wave speed, wavelength is inversely proportion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry Moseley
Henry Gwyn Jeffreys Moseley (; 23 November 1887 – 10 August 1915) was an English physicist, whose contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic number. This stemmed from his development of Moseley's law in X-ray spectra. Moseley's law advanced atomic physics, nuclear physics and quantum physics by providing the first experimental evidence in favour of Niels Bohr's theory, aside from the hydrogen atom spectrum which the Bohr theory was designed to reproduce. That theory refined Ernest Rutherford's and Antonius van den Broek's model, which proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. When World War I broke out in Western Europe, Moseley left his research work at the University of Oxford behind to volunteer for the Royal Engineers of the British Army. Moseley was assigned to the force o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kasimir Fajans
Kazimierz Fajans (Kasimir Fajans in many American publications; 27 May 1887 – 18 May 1975) was a Polish-Jewish physical chemist, a pioneer in the science of radioactivity and the co-discoverer of chemical element protactinium. Education and career Fajans was born May 27, 1887, in Warsaw, Congress Poland, to a family of Jewish background. After he had completed secondary school in Warsaw (1904), he studied chemistry in Germany, first at the University in Leipzig, and then in Heidelberg and Zürich. In 1909 he was awarded his PhD for research into the stereoselective synthesis of chiral compounds. In 1910 Fajans took a job at the laboratory of Ernest Rutherford in Manchester, where the nucleus was discovered. He then returned to Germany, where he became an assistant and subsequently assistant professor at the Technical University of Karlsruhe, researching radioactivity. In 1917 he headed the Faculty of Physical Chemistry at Munich University, and in 1932 became the Head of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oswald Helmuth Göhring
Oswald Helmuth Göhring, also known as Otto Göhring, (1889) was a German chemist who, with his teacher Kasimir Fajans, co-discovered the chemical element protactinium in 1913. Discovery of protactinium Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring at the University of Karlsruhe. The new element was named brevium due to the brief half-life of the isotope specific studied, Protactinium-234 (234 Pa). Fajans and Göhring also worked to identify as many isotopes of the new element as possible, and also to publicize their discovery—a process that was hampered by the beginning of World War I. In 1914, Göhring was conscripted into the army. Presumably he perished during the war; he is listed as the author of no further scientific articles or publications after 1915. A stable isotope of this element was discovered in 1918, and thus the name was changed to protoactinium, which was abbreviated in 1949 to its present name, protactinium. Pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protactinium
Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel. The element was first identified in 1913 by Kazimierz Fajans and Oswald Helmuth Göhring and named "brevium" because of the short half-life of the specific isotope studied, 234mPa. A more stable isotope of protactinium, 231Pa, was discovered in 191 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Biological Chemistry
The ''Journal of Biological Chemistry'' (''JBC'') is a weekly peer-reviewed scientific journal that was established in 1905., jbc.org Since 1925, it is published by the American Society for Biochemistry and Molecular Biology. It covers research in areas of biochemistry and molecular biology. The editor is Alex Toker. the journal is fully open access. In press articles are available free on its website immediately after acceptance. Editors The following individuals have served as editors of the journal: * 1906–1909: John Jacob Abel and Christian Archibald Herter * 1909–1910: Christian Archibald Herter * 1910–1914: Alfred Newton Richards * 1914–1925: Donald D. Van Slyke * 1925–1936: Stanley R. Benedict. After Benedict died, John T. Edsall served as temporary editor until the next editor was appointed. * 1937–1958: Rudolph J. Anderson * 1958–1967: John T. Edsall * 1968–1971: William Howard Stein * 1971–2011: Herbert Tabor * 2011–2015: Martha Fedor * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin A
Vitamin A is a fat-soluble vitamin that is an essential nutrient. The term "vitamin A" encompasses a group of chemically related organic compounds that includes retinol, retinyl esters, and several provitamin (precursor) carotenoids, most notably Β-Carotene, β-carotene (''beta''-carotene). Vitamin A has multiple functions: growth during embryo development, maintaining the immune system, and healthy vision. For aiding vision specifically, it combines with the protein opsin to form rhodopsin, the light-absorbing molecule necessary for both low-light (scotopic vision) and color vision. Vitamin A occurs as two principal forms in foods: A) retinoids, found in Animal source foods, animal-sourced foods, either as retinol or bound to a fatty acid to become a retinyl ester, and B) the carotenoids Α-Carotene, α-carotene (''alpha''-carotene), β-carotene, Γ-Carotene, γ-carotene (''gamma''-carotene), and the xanthophyll beta-cryptoxanthin (all of which contain β-ionone rings) that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |