|
Batch Distillation
Batch distillation refers to the use of distillation in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated. This is in contrast with continuous distillation where the feedstock is added and the distillate drawn off without interruption. Batch distillation has always been an important part of the production of seasonal, or low capacity and high-purity chemicals. It is a very frequent separation process in the pharmaceutical industry. Batch rectifier The simplest and most frequently used batch distillation configuration is the batch rectifier, including the alembic and pot still. The batch rectifier consists of a pot (or reboiler), rectifying column, a condenser, some means of splitting off a portion of the condensed vapour (distillate) as reflux, and one or more receivers. The pot is filled with liquid mixture and heated. Vapour flows upwards in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or Cracking (chemistry), cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In Chemical industry, industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Distillation
Vacuum distillation is distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation. Laboratory-scale applications Compounds with a boiling point lower than 150 °C typically are distilled at ambient pressure. For samples with high boiling points, short-path distillation apparatus is commonly employed. This technique is amply illustrated in Organic Synthesis. Rotary evaporation Rotary evaporation is a common technique used in laboratories to concentrate or isolate a compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Distillation
Steam distillation is a separation process that consists in distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container. If, as is usually the case, the volatiles are not miscible with water, they will spontaneously form a distinct phase after condensation, allowing them to be separated by decantation or with a separatory funnel. Steam distillation can be used when the boiling point of the substance to be extracted is higher than that of water, and the starting material cannot be heated to that temperature because of decomposition or other unwanted reactions. It may also be useful when the amount of the desired substance is small compared to that of the non-volatile residues. It is often used to separate volatile essential oils from plant materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroazeotrope
A heteroazeotrope is an azeotrope where the vapour phase coexists with two liquid phases. Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture Examples of heteroazeotropes *Benzene - Water NBP 69.2 °C *Dichloromethane - Water NBP 38.5 °C * n-Butanol - Water NBP 93.5 °C * Toluene - Water NBP 82 °C Continuous heteroazeotropic distillation Heterogeneous distillation means that during the distillation the liquid phase of the mixture is immiscible. In this case on the plates can be two liquid phases and the top vapour condensate splits in two liquid phases, which can be separated in a decanter. The simplest case of continuous heteroazeotropic distillation is the separation of a binary heterogeneous azeotropic mixture. In this case the system contains two columns and a decanter. The fresh feed (A-B) is added into the first column. (The feed may also be added into the decanter directly or into the second column depending on the composition of the mixture). Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil. Laboratory setup Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a Bunsen burner, a round-bottomed flask and a condenser, as well as the single-purpose fractionating column. As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at while water boils at . So, by heating the mixture, the most volatile component (ethanol) will concen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extractive Distillation
Extractive distillation is defined as distillation in the presence of a miscible, high-boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity. Such mixtures cannot be separated by simple distillation, because the volatility of the two components in the mixture is nearly the same, causing them to evaporate at nearly the same temperature at a similar rate, making normal distillation impractical. The method of extractive distillation uses a separation solvent, which is generally non-volatile, has a high boiling point and is miscible with the mixture, but doesn't form an azeotropic mixture. The solvent interacts differently with the components of the mixture thereby causing their relative volatilities to change. This enables the new three-part mixture to be separated by normal distillation. The original component with the gre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azeotropic Distillation
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, ''azeotropic distillation'' usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous (e.g. producing two, immiscible liquid phases), such as the example below with the addition of benzene to water and ethanol. This practice of adding an entrainer which forms a separate phase is a specific sub-set of (industrial) azeotropic distillation methods, or combination thereof. In some senses, adding an entrainer is similar to extractive distillation. Material separation agent The addition of a material separation agent, such as benzene to an ethanol/water mixture, changes the molecular interactions and eliminates the azeotrope. Added in the liquid phase, the new component can alter the activity coefficient of various compounds in different ways thus altering a mixture's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
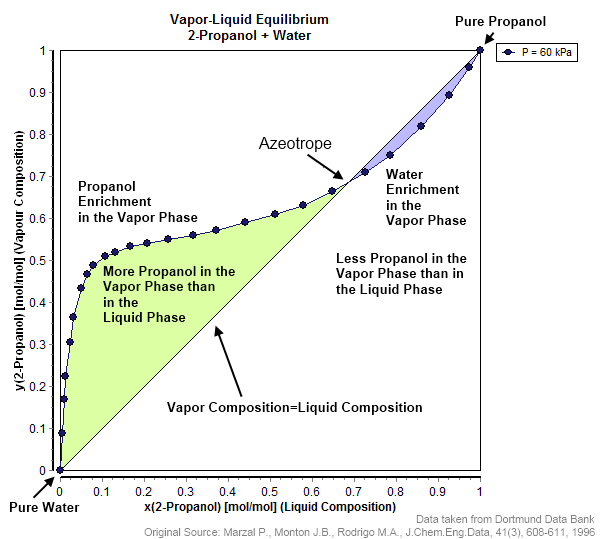
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called (especially in older texts) ''constant boiling point mixtures''. Some azeotropic mixtures of pairs of compounds are known, and many azeotropes of three or more compounds are also known. In such a case it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. There are two types of azeotropes: minimum boiling azeotrope and maximum boiling azeotrope. A Solution (chemistry), solution that shows greater positive deviation from Raoult's law forms a minimum boiling azeotrope at a speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Separation
Phase separation is the creation of two distinct phases from a single homogeneous mixture. The most common type of phase separation is between two immiscible liquids, such as oil and water. Colloids are formed by phase separation, though not all phase separations forms colloids - for example oil and water can form separated layers under gravity rather than remaining as microscopic droplets in suspension. Phase separation in cold gases A mixture of two helium isotopes (helium-3 and helium-4) in a certain range of temperatures and concentrations separates into parts. The initial mix of the two isotopes spontaneously separates into ^He-rich and ^3He-rich regions. Phase separation also exists in ultracold gas systems. It has been shown experimentally in a two-component ultracold Fermi gas case. The phase separation can compete with other phenomena as vortex lattice formation or an exotic Fulde-Ferrell-Larkin-Ovchinnikov phase. See also * Biomolecular condensate * Collo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
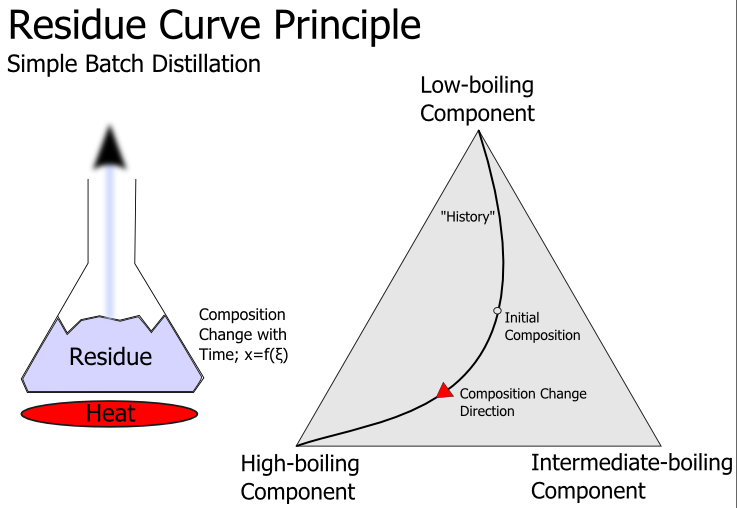
Residue Curve
A residue curve describes the change of the composition of the liquid phase of a chemical mixture during continuous evaporation at the condition of vapor–liquid equilibrium (open distillation). Multiple residue curves for a single system are called ''residue curves map''. Residue curves allow testing the feasibility of a separation of mixtures and therefore are a valuable tool in designing distillation processes. Residue curve maps are typically used for examining ternary mixtures which can't be easily separated by distillation because of azeotropic points or too small relative volatilities. Characteristics # Residue curves start at the composition of a feed and then move to pure components or azeotropic points with higher temperatures (isobaric condition) or lower vapor pressures (isothermal condition). This happens because more of the light boiling substances are vaporized than of the high boiling substances and therefore the concentration of the high boilers increase in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |