|
Ammonia Production
Ammonia is one of the most highly produced inorganic chemicals. There are numerous large-scale ammonia plants worldwide, producing a grand total of 144 million tonnes of nitrogen (equivalent to 175 million tonnes of ammonia) in 2016. This has increased to 235 million tonnes of ammonia in 2021. China produced 31.9% of the worldwide production, followed by Russia with 8.7%, India with 7.5%, and the United States with 7.1%. 80% or more of the ammonia produced is used for fertilizing agricultural crops. Ammonia is also used for the production of plastics, fibres, explosives, nitric acid (via the Ostwald process), and intermediates for dyes and pharmaceuticals. History Dry distillation Before the start of World War I, most ammonia was obtained by the dry distillation of nitrogenous vegetable and animal products; by the reduction of nitrous acid and nitrites with hydrogen; and also by the decomposition of ammonium salts by alkaline hydroxides or by quicklime, the salt most generall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquified Petroleum Gas
Liquefied petroleum gas (LPG or LP gas) is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane and n-butane. LPG is used as a fuel gas in heating appliances, cooking equipment, and vehicles. It is increasingly used as an aerosol propellant and a refrigerant, replacing chlorofluorocarbons in an effort to reduce damage to the ozone layer. When specifically used as a vehicle fuel, it is often referred to as autogas or even just as gas. Varieties of LPG that are bought and sold include mixes that are mostly propane (), mostly butane (), and, most commonly, mixes including both propane and butane. In the northern hemisphere winter, the mixes contain more propane, while in summer, they contain more butane. In the United States, mainly two grades of LPG are sold: commercial propane and HD-5. These specifications are published by the Gas Processors Association (GPA) and the American Society of Testing and M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
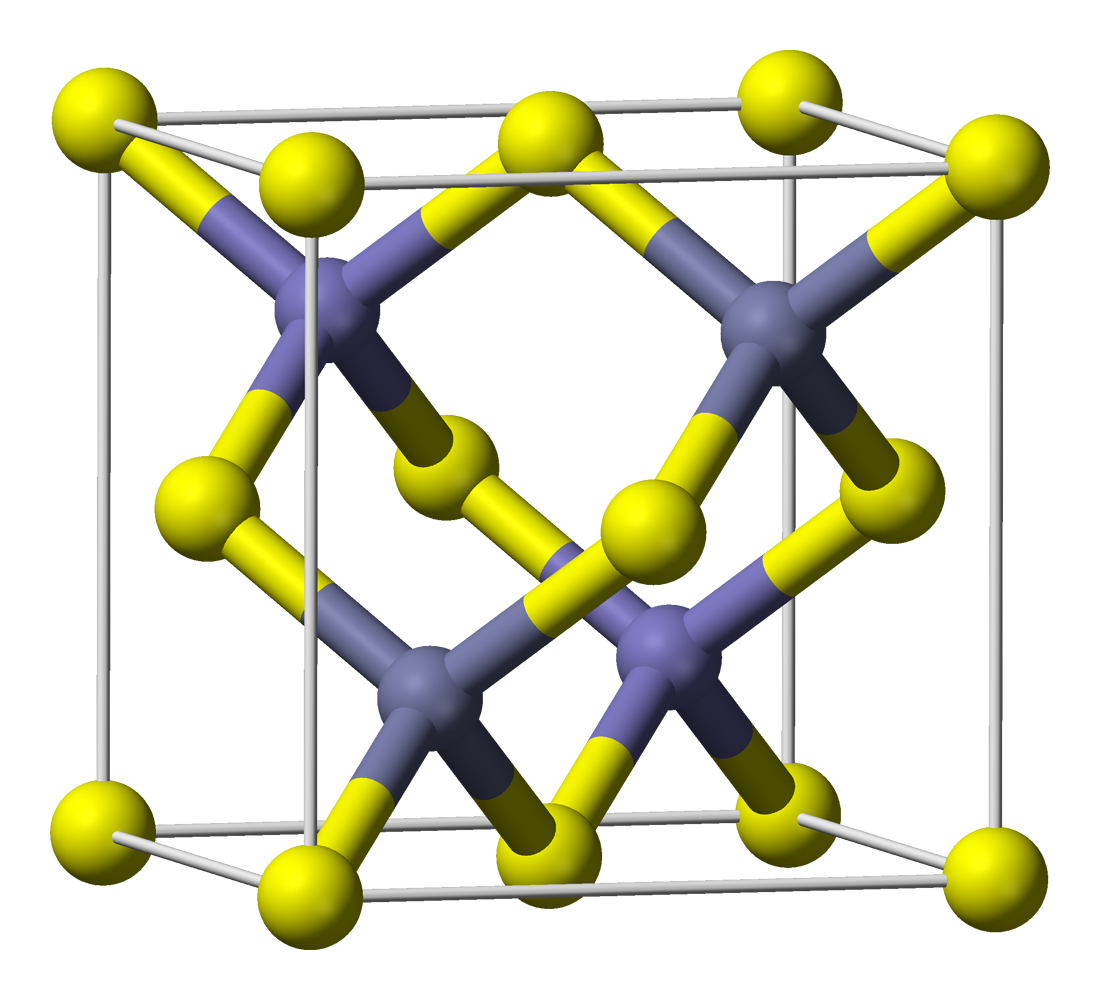
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Appli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. ZnO is a wide-band gap semiconductor of the II-VI semiconductor group. The native doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility, wide band gap, and strong room-temperature luminescence. Those properties make ZnO valuable for a variety of emerging applications: transparent electrodes in liquid crystal displays, energy-saving or heat-protecting windows, and electronics as thin-film transistors and lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is ''stinkdamp''. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. The British English spelling of this compound is hydrogen sulphide, a spelling no longer recommended by the Royal Society of Chemistry or the International Union of Pure and Applied Chemistry. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or it or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)