|
Andersen Thermostat
The Andersen thermostat is a proposal in molecular dynamics simulation for maintaining constant temperature conditions. It is based on periodic reassignment of the velocities of atoms or molecules. For each atom or molecule, the reassigned velocity Velocity is a measurement of speed in a certain direction of motion. It is a fundamental concept in kinematics, the branch of classical mechanics that describes the motion of physical objects. Velocity is a vector (geometry), vector Physical q ... is picked randomly according to Maxwell–Boltzmann statistics for the given temperature. The thermostat is named after chemist from his 1980 work on the topic. Introduction When a system exists at some temperature, the energy of particles' degrees of freedom are randomly distributed according to a Boltzmann distribution. The energy of such systems is not constant; it's constantly fluctuating due to exchange of energy with surroundings. The Andersen thermostat models this exchange of e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the Motion (physics), physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamics (mechanics), dynamic "evolution" of the system. In the most common version, the trajectory, trajectories of atoms and molecules are determined by Numerical integration, numerically solving Newton's laws of motion, Newton's equations of motion for a system of interacting particles, where Force (physics), forces between the particles and their potential energy, potential energies are often calculated using interatomic potentials or molecular mechanics, molecular mechanical Force field (chemistry), force fields. The method is applied mostly in chemical physics, materials science, and biophysics. Because molecular systems typically consist of a vast number of particles, it is impossible to determine the properties of such complex systems analyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Velocity
Velocity is a measurement of speed in a certain direction of motion. It is a fundamental concept in kinematics, the branch of classical mechanics that describes the motion of physical objects. Velocity is a vector (geometry), vector Physical quantity, quantity, meaning that both magnitude and direction are needed to define it. The Scalar (physics), scalar absolute value (Magnitude (mathematics), magnitude) of velocity is called , being a coherent derived unit whose quantity is measured in the International System of Units, SI (metric system) as metres per second (m/s or m⋅s−1). For example, "5 metres per second" is a scalar, whereas "5 metres per second east" is a vector. If there is a change in speed, direction or both, then the object is said to be undergoing an ''acceleration''. Definition Average velocity The average velocity of an object over a period of time is its Displacement (geometry), change in position, \Delta s, divided by the duration of the period, \Delt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
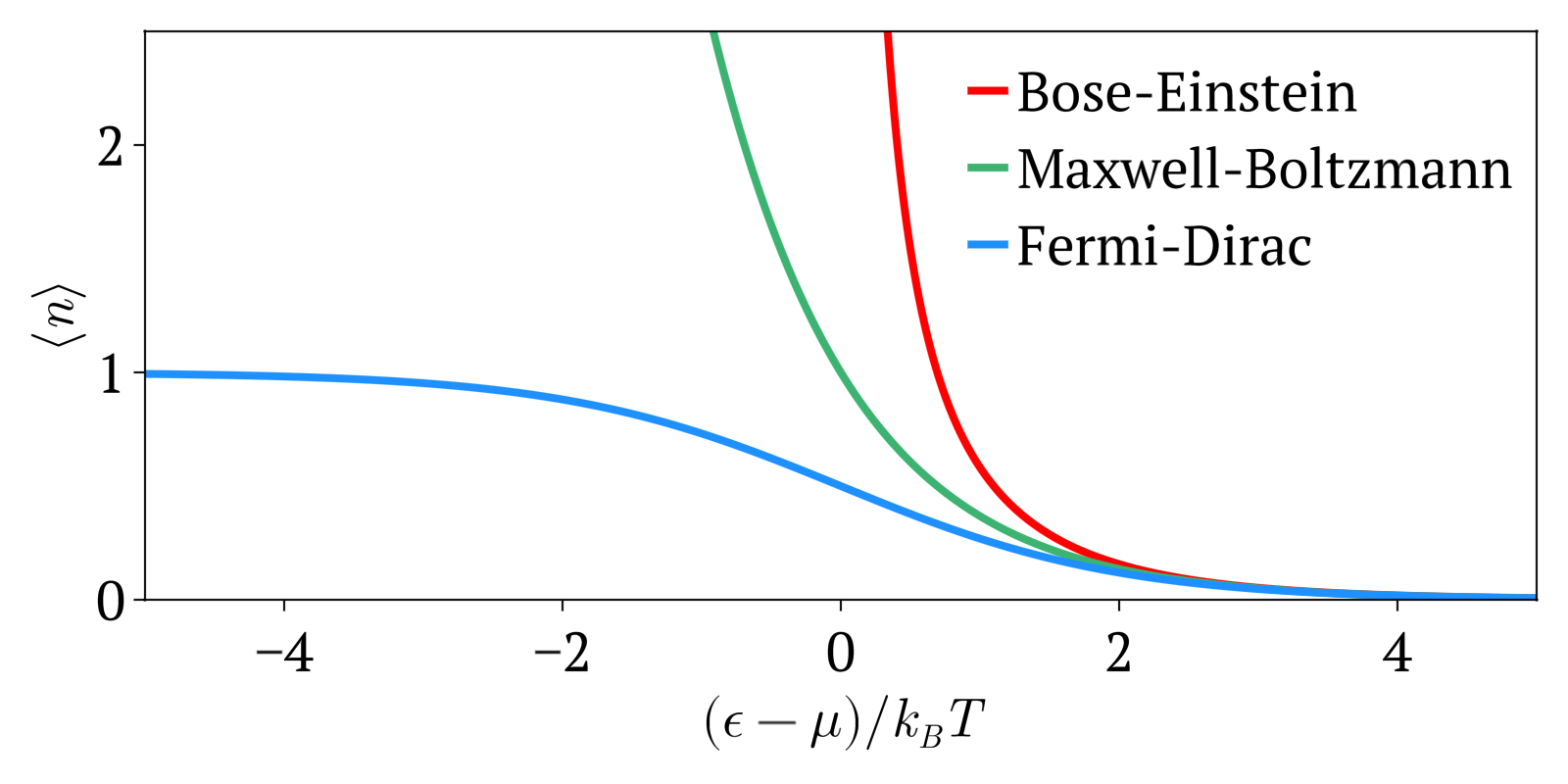
Maxwell–Boltzmann Statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density is low enough to render quantum effects negligible. The expected number of particles with energy \varepsilon_i for Maxwell–Boltzmann statistics is : \langle N_i \rangle = \frac = \frac\,g_i e^, where: * \varepsilon_i is the energy of the ''i''th energy level, * \langle N_i \rangle is the average number of particles in the set of states with energy \varepsilon_i, * g_i is the degeneracy of energy level ''i'', that is, the number of states with energy \varepsilon_i which may nevertheless be distinguished from each other by some other means,For example, two simple point particles may have the same energy, but different momentum vectors. They may be distinguished from each other on this basis, and the degeneracy will be the number of po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nosé–Hoover Thermostat
The Nosé–Hoover thermostat is a deterministic algorithm for constant-temperature molecular dynamics simulations. It was originally developed by Shuichi Nosé and was improved further by William G. Hoover. Although the heat bath of Nosé–Hoover thermostat consists of only one imaginary particle, simulation systems achieve realistic constant-temperature condition ( canonical ensemble). Therefore, the Nosé–Hoover thermostat has been commonly used as one of the most accurate and efficient methods for constant-temperature molecular dynamics simulations. Introduction In classical molecular dynamics, simulations are done in the microcanonical ensemble; a number of particles, volume, and energy have a constant value. In experiments, however, the temperature is generally controlled instead of the energy. The ensemble of this experimental condition is called a canonical ensemble. Importantly, the canonical ensemble is different from microcanonical ensemble from the viewpoint of st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |