|
Aldehyde Dehydrogenase
Aldehyde dehydrogenases () are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes (R–C(=O)) to carboxylic acids (R–C(=O)). The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes. Function Aldehyde dehydrogenase is a polymorphic enzyme responsible for the oxidation of aldehydes to carboxylic acids. There are three different classes of these enzymes in mammals: class 1 (low ''K''m, cytosolic), class 2 (low ''K''m, mitochondrial), and class 3 (high ''K''m, such as those expressed in tumors, stomach, and cornea). In all three classes, constitutive and inducible forms exist. ALDH1 and ALDH2 are the most important enzymes for aldehyde oxidation, and both are tetrameric enzymes composed of 54 kDa subunits. These enzymes are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ALDH2
Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ''ALDH2'' gene located on chromosome 12. ALDH2 belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. ALDH2 has a low Km for acetaldehyde, and is localized in mitochondrial matrix. The other liver isozyme, ALDH1, localizes to the cytosol. Most White people have both major isozymes, while approximately 36% of East Asians have the cytosolic isozyme but not a functional mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among East Asians than among Whites could be related to this absence of a catalytically active form of ALDH2. The increased exposure to acetaldehyde in individuals with the catalytically inactive form may also confer greater susceptibility to many types of cancer. Gene The ''ALDH2'' gene is about 44 kbp in length and contains at least 13 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other, nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In cellular metabolism, NAD is involved in redox reactions, carrying electrons from one reaction to another, so it is found in two forms: NAD is an oxidizing agent, accepting electrons from other molecules and becoming reduced; with H+, this reaction forms NADH, which can be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. It is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to or from proteins, in posttranslational modifications. Because of the import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noradrenaline Breakdown
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" (from Latin '' ad'', "near", and '' ren'', "kidney") is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" (from Ancient Greek ἐπῐ́ (''epí''), "upon", and νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rises during wakefulness, and reaches much higher levels during situations of stress or danger, in the so-c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Durian
The durian () is the edible fruit of several tree species belonging to the genus ''Durio''. There are 30 recognized species, at least nine of which produce edible fruit. ''Durio zibethinus'', native to Borneo and Sumatra, is the only species available on the international market. It has over 300 named varieties in Thailand and over 200 in Malaysia as of 2021. Other species are sold in their local regions. Known in some regions as the "king of fruits", the durian is distinctive for its large size, strong odour, and Spine (botany), thorn-covered peel (fruit), rind. The fruit can grow as large as long and in diameter, and it typically weighs . Its shape ranges from oblong to round, the colour of its husk from green to brown, and its flesh from pale yellow to red, depending on the species. Some people regard the durian as having a pleasantly sweet fragrance, whereas others find the aroma overpowering and unpleasant. The persistence of its strong odour, which may linger for sev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Tsukuba
is a List of national universities in Japan, national research university located in Tsukuba, Ibaraki Prefecture, Ibaraki, Japan. The university has 28 college clusters and schools with around 16,500 students (as of 2014). The main Tsukuba campus covers an area of 258 hectares (636 acres), making it the second largest single campus in Japan. The university has a branch campus in Bunkyō, Bunkyo-ku, Tokyo, offering graduate programs for working adults in the capital and managing K-12 schools in Tokyo that are attached to the university. Three Nobel Prize laurates have taught at the university, Leo Esaki, Hideki Shirakawa and Sin-Itiro Tomonaga. Apart from them, Satoshi Ōmura studied as an audit student. History The University of Tsukuba can trace its roots back to , a normal school established in 1872 to educate primary and secondary teachers. The school was promoted to a university in 1929, as Tokyo University of Literature and Science (東京文理科大学, ''Tōkyō ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
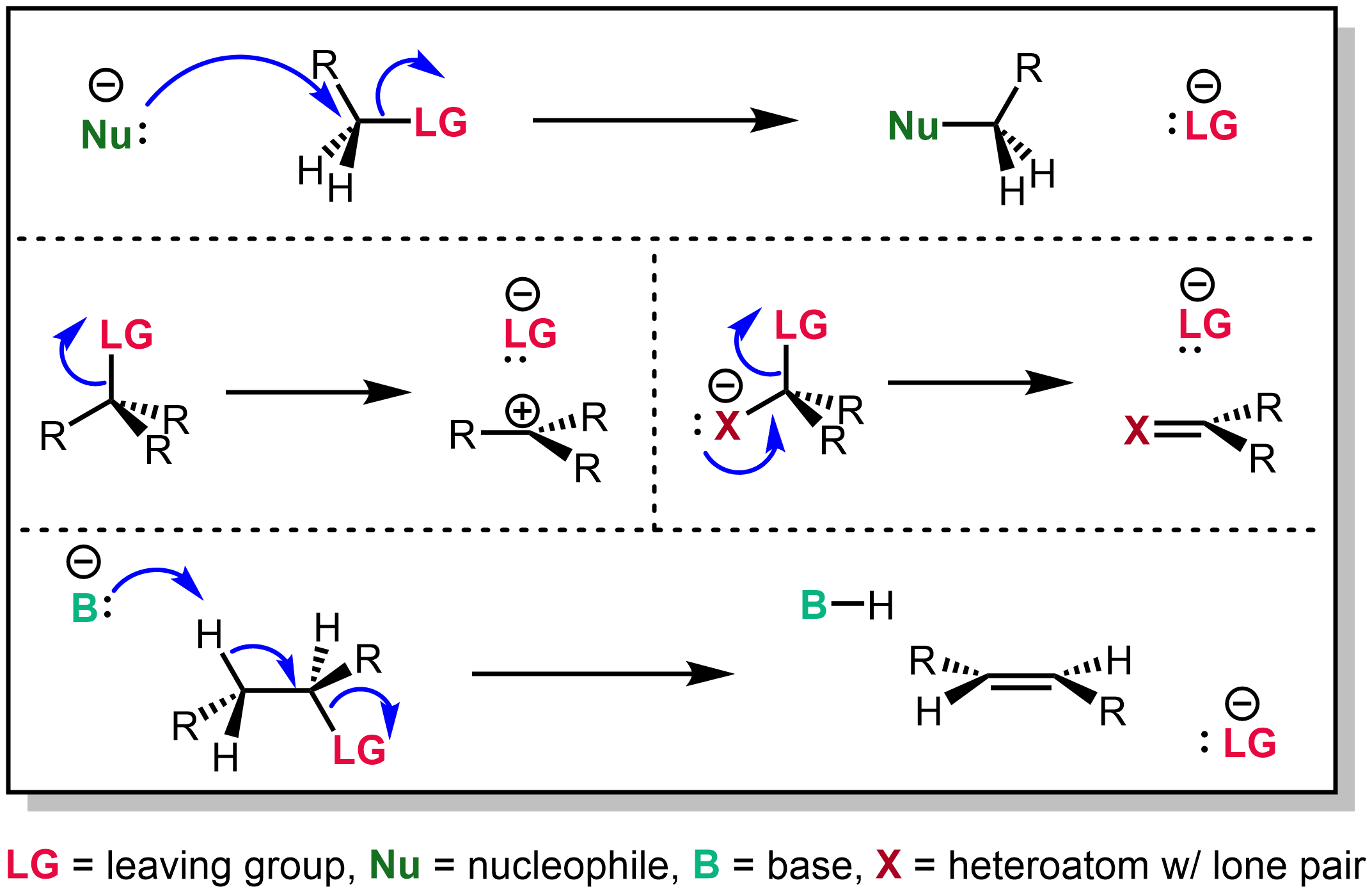
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NAD(P)H
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other, nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In cellular metabolism, NAD is involved in redox reactions, carrying electrons from one reaction to another, so it is found in two forms: NAD is an oxidizing agent, accepting electrons from other molecules and becoming reduced; with H+, this reaction forms NADH, which can be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. It is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to or from proteins, in posttranslational modifications. Because of the importa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all chemical compound, compounds containing covalent bond, covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative chemical element, element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form Binary compounds of hydrogen, binary compounds with hydrogen, the exceptions being helium, He, neon, Ne, argon, Ar, krypton, Kr, promethium, Pm, osmium, Os, iridium, Ir, radon, Rn, francium, Fr, and radium, Ra. exotic atom#exotic molecules, Exotic molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, Hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleophilicity is a kinetic property ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |