|
1,1'-Ferrocenediisocyanate
1,1'-Ferrocenediisocyanate (1,1'-diisocyanatoferrocene) is the organoiron compound with the formula . It is the simplest di isocyanate derivative of ferrocene. It can be synthesized by the Curtius rearrangement of the diacyl azide, using several protocols starting from 1,1'-ferrocenedicarboxylic acid. The compound is useful as an intermediate in the synthesis of 1,1'-diaminoferrocene by hydrolysis of the isocyanates. Various poly( siloxane–urethane) crosslinked polymers can be formed by reaction with siloxane-diols. These compounds are of interest as electrochemically active polymers that might have good mechanical properties A materials property is an intensive property of a material, i.e., a physical property that does not depend on the amount of the material. These quantitative properties may be used as a metric by which the benefits of one material versus another ca ... at low temperature. References Ferrocenes Cyclopentadienyl complexes Isocyanates {{organi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1'-diaminoferrocene
1,1'-Diaminoferrocene is the organoiron compound with the formula . It is the simplest diamine derivative of ferrocene. It is a yellow, air-sensitive solid that is soluble in aqueous acid. The 1,1' part of its name refers to the location of the amine groups on separate rings. Compared to the parent ferrocene, the diamine is about 600 mV more reducing. It can be prepared from the diisocyanate , which in turn is derived from 1,1'-ferrocenedicarboxylic acid. 1,1'-Diaminoferrocene was originally prepared by hydrogenation of 1,1'-diazidoferrocene ( ). 1,1'-Diaminoferrocene has been incorporated into various diamide and diimine ligands, which form catalyst Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...s that exhibit redox switching. References {{DEFAULTSORT:Diaminoferrocen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1'-ferrocenedicarboxylic Acid
1,1'-Ferrocenedicarboxylic acid is the organoiron compound with the formula . It is the simplest dicarboxylic acid derivative of ferrocene. It is a yellow solid that is soluble in aqueous base. The 1,1' part of its name refers to the location of the carboxylic acid groups on separate rings. It can be prepared by hydrolysis of its diesters (R = Me, Et), which in turn are obtained by treatment of ferrous chloride with the sodium salt of the carboxyester of cyclopentadienide . Ferrocenedicarboxylic acid is the precursor to many derivatives such as the diacid chloride, the diisocyanate, the diamide, and diamine, respectively, , , , and . Derivatives of ferrocenedicarboxylic acid are components of some redox switches and redox active coatings. Related compounds * Ferrocenecarboxylic acid Ferrocenecarboxylic acid is the organoiron compound with the formula . It is the simplest carboxylic acid derivative of ferrocene. It can be prepared in two steps from ferrocene by ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoiron Compound
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and " greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well. Iron(0) and more reduced states Carbonyl complexes Important iron carbonyls are the three neutral binary carbonyls, iron pentacarbonyl, diiron nonacarbonyl, and triiron dodecacarbonyl. One or more carbonyl ligands ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crosslink
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Properties
A materials property is an intensive property of a material, i.e., a physical property that does not depend on the amount of the material. These quantitative properties may be used as a metric by which the benefits of one material versus another can be compared, thereby aiding in materials selection. A property may be a constant or may be a function of one or more independent variables, such as temperature. Materials properties often vary to some degree according to the direction in the material in which they are measured, a condition referred to as anisotropy. Materials properties that relate to different physical phenomena often behave linearly (or approximately so) in a given operating range. Modeling them as linear functions can significantly simplify the differential constitutive equations that are used to describe the property. Equations describing relevant materials properties are often used to predict the attributes of a system. The properties are measured by standar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal dio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siloxane
A siloxane is a functional group in organosilicon chemistry with the Si−O−Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)''n''OH and (OSiH2)n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen (O2-) atom. The siloxane functional group forms the backbone of silicones, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− (where the three Rs may be different) is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility. Structure Siloxanes generally adopt structures expected for linked tetrahedral ("''sp''3-like") centers. The Si−O bond length is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si-O-Si angle is rather open at 142.5°. By contrast, the C−O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
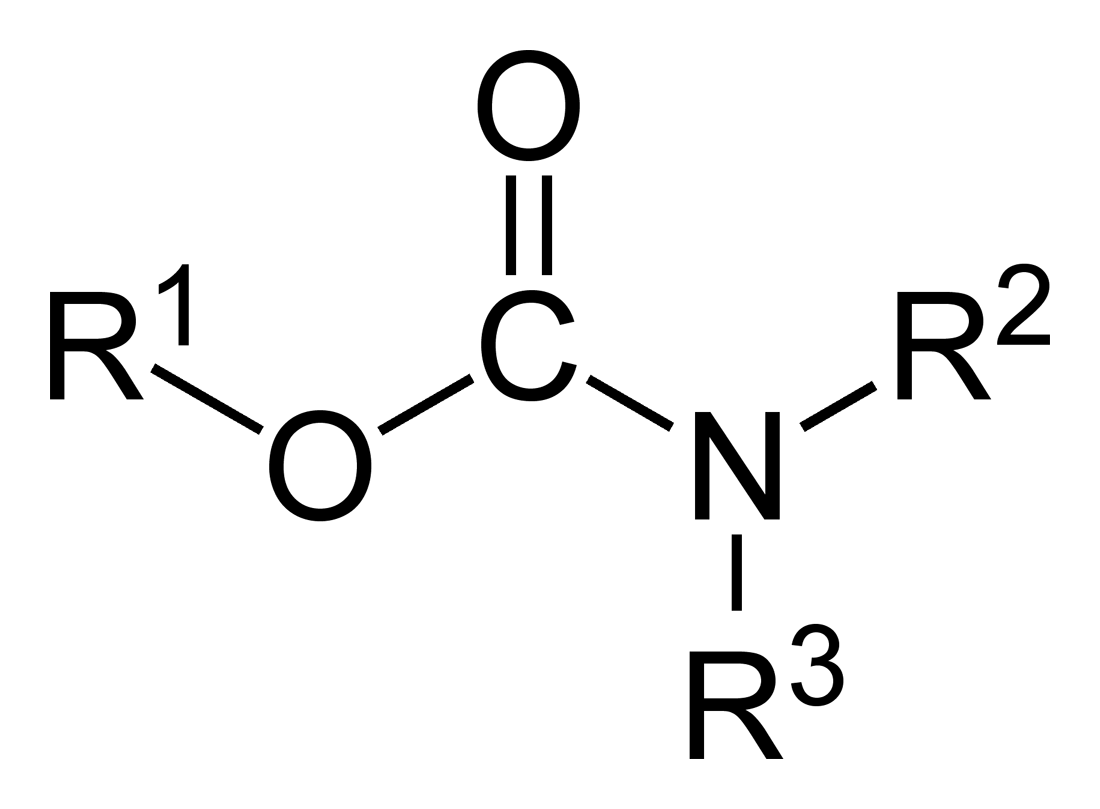
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C-N=C angle is 134.9° and the N=C=O angle is 173.1° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |