|
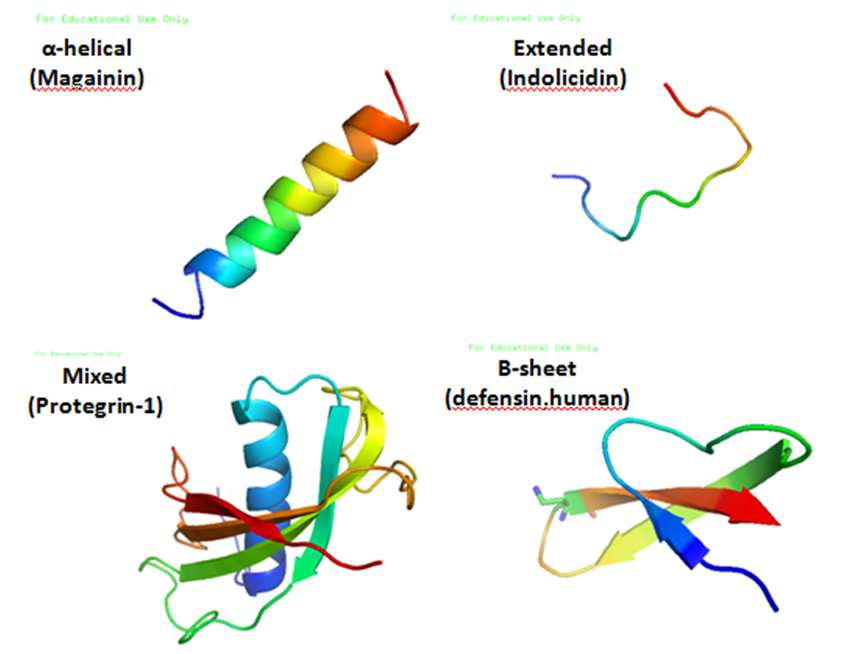
β-defensin
Beta defensins are a family of vertebrate defensins. The beta defensins are antimicrobial peptides implicated in the resistance of epithelial surfaces to microbial colonization. Defensins are 2 to 6 kDa, cationic, microbicidal peptides active against many Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses, containing three pairs of intramolecular disulfide bonds. On the basis of their size and pattern of disulfide bonding, mammalian defensins are classified into alpha, beta and theta categories. Every mammalian species explored thus far has beta-defensins. In cows, as many as 13 beta-defensins exist in neutrophils. However, in other species, beta-defensins are more often produced by epithelial cells lining various organs (e.g. the epidermis, bronchial tree and genitourinary tract). Human, rabbit and guinea-pig beta-defensins, as well as human beta-defensin-2 (hBD2), induce the activation and degranulation of mast cells, resulting in the release of histamine a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Defensin
Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct Antimicrobial, antimicrobial activity, Immune system, immune signaling activities, or both. They are variously active against bacteria, fungus, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds. In animals, they are produced by cells of the innate immune system and epithelial cells, whereas in plants and fungi they are produced by a wide variety of tissues. An organism usually produces many different defensins, some of which are stored inside the cells (e.g. in neutrophil granulocytes to kill Phagocyte, phagocytosed bacteria), and others are secreted into the extracellular medium. For those that directly kill microbes, their mechanism of action varies from disruption of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Peptides
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between Prokaryote, prokaryotic and eukaryota, eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Defensin
Alpha defensins are a family of mammalian defensin peptides of the alpha subfamily. They are also known as cryptdins and are produced within the small bowel. ''Cryptdin'' is a portmanteau of ''crypt'' and ''defensin''. Defensins are 2-6 kDa, cationic, antimicrobial peptides active against many Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses, containing three pairs of intramolecular disulfide bonds. On the basis of their size and pattern of disulfide bonding, mammalian defensins are classified into alpha, beta and theta categories. Alpha-defensins, which have been identified in humans, monkeys and several rodent species, are particularly abundant in neutrophils, certain macrophage populations and Paneth cells of the small intestine. Defensins are produced constitutively and/or in response to microbial products or proinflammatory cytokines. Some defensins are also called corticostatins because they inhibit corticotropin-stimulated corticosteroid product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theta Defensin
Theta-defensins (θ-defensins, retrocyclins, or demidefensins) are a family of mammalian antimicrobial peptides. They are found in non-human 'Old World' primates, but not in human, gorilla, bonobo, and chimpanzee. Structure θ-defensins are cyclic peptides of 18 amino acids (~2 kDa), possessing antimicrobial activity against a range of Gram-positive and Gram-negative bacteria, fungi, and some retroviruses. They consist of a pair of antiparallel β-sheets linked by three disulfide bonds arranged as a ladder along the sheets to form an extremely stable structure. Additionally, the peptides may self-associate into trimers. Biosynthesis In rhesus macaque (''Macaca mulatta'') and olive baboon (''Papio anubis''), θ-defensins are produced from precursor proteins with 76 amino acids each. A single nine amino acid peptide is derived from each precursor. Two of these nine amino acid peptides are spliced together to form the circular 18 amino acid defensin. Since there are two precur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups (Alpha and beta carbon, alpha- , beta- , gamma- (γ-) amino acids, etc.); other categories relate to Chemical polarity, polarity, ionization, and side-chain group type (aliphatic, Open-chain compound, acyclic, aromatic, Chemical polarity, polar, etc.). In the form of proteins, amino-acid ''Residue (chemistry)#Biochemistry, residues'' form the second-largest component (water being the largest) of human muscles and other tissue (biology), tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonds
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a more electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), orbital interactions, and quantum mechanical delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxygen (O), and fluorine (F), due to their high electronegativity and ability to engage in stronger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex or protein multimer, multimer formed by two protein monomers, or single proteins, which are usually Non-covalent interaction, non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", ''wikt:di-#Prefix, di-'' + ''wikt:-mer#Suffix, -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein IKBKG, NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physics), work needed to move a test charge from a reference point to a specific point in a static electric field. The test charge used is small enough that disturbance to the field is unnoticeable, and its motion across the field is supposed to proceed with negligible acceleration, so as to avoid the test charge acquiring kinetic energy or producing radiation. By definition, the electric potential at the reference point is zero units. Typically, the reference point is Earth (electricity), earth or a point at infinity, although any point can be used. In classical electrostatics, the electrostatic field is a vector quantity expressed as the gradient of the electrostatic potential, which is a scalar (physics), scalar quantity denoted by or occasi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toll-like Receptors
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane protein, single-spanning receptor (biochemistry), receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from Microorganism, microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in Intracellular receptor, intracellular Vesicle (biology and chemistry), vesicles (because they are sensors of nucleic acids). TLRs received their name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipoteichoic Acid
Lipoteichoic acid (LTA) is a major constituent of the cell wall of gram-positive bacteria. These organisms have an inner (or cytoplasmic) membrane and, external to it, a thick (up to 80 nanometer) peptidoglycan layer. The structure of LTA varies between the different species of gram-positive bacteria and may contain long chains of ribitol or glycerol phosphate. LTA is anchored to the cell membrane via a diacylglycerol. It acts as regulator of autolytic wall enzymes ( muramidases). It has antigenic properties being able to stimulate specific immune response. LTA may bind to target cells non-specifically through membrane phospholipids, or specifically to CD14 and to Toll-like receptors. Binding to TLR-2 has shown to induce NF-κB expression(a central transcription factor In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription (genetics), transcription of genetics, genetic informat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Biological membranes include cell membranes (outer coverings of cells or organelles that allow passage of certain constituents); nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucous membrane, mucosae and serous membrane, serosae. Synthetic membranes are made by humans for use in laboratory, laboratories and industry (such as chemical plants). This concept of a membrane has been known since the eighteenth century but was used little outside of the laboratory until the end of World War II. Drinking water supplies in Europe had been compromised by The War and membrane filters were used to test for water safety. However, due to the lack of reliability, slow operation, reduced selectivity and elevated costs, membran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytokines
Cytokines () are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes and mast cells, as well as Endothelium, endothelial cells, fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell. Due to their size, cytokines cannot cross the lipid bilayer of cells to enter the cytoplasm and therefore typically exert their functions by interacting with specific cytokine receptor, cytokine receptors on the target cell surface. Cytokines are especially important in the immune system; cytokines modulate the balance between humoral immunity, humoral and cell-mediated immunity, cell-based immune responses, and they regulate the maturation, growth, and responsiveness of particular cell populations. Some cytokines enhance or inhibit the action of other cytokines in complex way ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |