|
α-Ketoglutaric Acid
α-Ketoglutaric acid is an organic compound with the formula ). A white, nontoxic solid, it is a common dicarboxylic acid. Relevant to its biological roles, it exists in water as its conjugate base α-ketoglutarate. It is also classified as a 2-ketocarboxylic acid. β-Ketoglutaric acid is an isomer. "Ketoglutaric acid" and "ketoglutarate", when not qualified as α or β, almost always refers respectively to α-ketoglutaric acid or α-ketoglutarate. α-Ketoglutarate is an intermediate in the citric acid cycle, a cycle that supplies the energy to cells. It is also an intermediate in or product of several other metabolic pathways. These include its being a component of metabolic pathways that: make amino acids and in the process regulate the cellular levels of carbon, nitrogen, and ammonia; reduce the cellular levels of potentially toxic reactive oxygen species; and synthesize the neurotransmitter gamma-aminobutyric acid. It also acts as a direct stimulator of, or cofactor ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
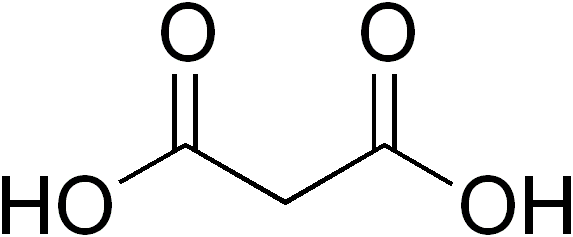
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common or illustrative examples : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T Cells
T cells (also known as T lymphocytes) are an important part of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface. T cells are born from hematopoietic stem cells, found in the bone marrow. Developing T cells then migrate to the thymus gland to develop (or mature). T cells derive their name from the thymus. After migration to the thymus, the precursor cells mature into several distinct types of T cells. T cell differentiation also continues after they have left the thymus. Groups of specific, differentiated T cell subtypes have a variety of important functions in controlling and shaping the immune response. One of these functions is immune-mediated cell death, and it is carried out by two major subtypes: CD8+ "killer" (cytotoxic) and CD4+ "helper" T cells. (These are named for the presence of the cell surface proteins CD8 or CD4.) CD8+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutaminolysis
Glutaminolysis (''glutamine'' + ''wikt:-lysis, -lysis'') is a series of biochemical reactions by which the amino acid glutamine is wikt:lyse#Verb, lysed to glutamic acid, glutamate, aspartate, CO2, pyruvic acid, pyruvate, Lactic acid, lactate, alanine and citric acid, citrate. The glutaminolytic pathway Glutaminolysis partially recruits reaction steps from the citric acid cycle and the malate-aspartate shuttle. Reaction steps from glutamine to α-ketoglutarate The conversion of the amino acid glutamine to α-ketoglutarate takes place in two reaction steps: 1. Hydrolysis of the amino group of glutamine yielding glutamate and ammonium. Catalyzing enzyme: glutaminase (EC 3.5.1.2) 2. Glutamate can be excreted or can be further metabolized to α-ketoglutarate. For the conversion of glutamate to α-ketoglutarate three different reactions are possible: Catalyzing enzymes: *glutamate dehydrogenase (GlDH), EC 1.4.1.2 *glutamate pyruvate transaminase (GPT), also called alanine trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxoglutarate Dehydrogenase Complex
The oxoglutarate dehydrogenase complex (OGDC) or α-ketoglutarate dehydrogenase complex is an enzyme complex, most commonly known for its role in the citric acid cycle. Units Much like pyruvate dehydrogenase complex (PDC), this enzyme forms a complex composed of three components: Three classes of these multienzyme complexes have been characterized: one specific for pyruvate, a second specific for 2-oxoglutarate, and a third specific for branched-chain α-keto acids. The oxoglutarate dehydrogenase complex has the same subunit structure and thus uses the same cofactors as the pyruvate dehydrogenase complex and the branched-chain alpha-keto acid dehydrogenase complex (TPP, CoA, lipoate, FAD and NAD). Only the E3 subunit is shared in common between the three enzymes. Properties Metabolic pathways This enzyme participates in three different pathways: * Citric acid cycle (KEGG linkMAP00020 * Lysine degradation (KEGG linkMAP00310 * Tryptophan metabolism (KEGG linkMAP00380 Kine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocitrate Dehydrogenase
Isocitrate dehydrogenase (IDH) () and () is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome. Structure The NAD-IDH is composed of three subunits, is allosterically regulated, and requires an integrated Mg2+ or Mn2+ ion. The closest homologue that has a known structure is the '' E. coli'' NADP-dependent IDH, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle. Properties Oxaloacetic acid undergoes successive deprotonations to give the dianion: :HO2CC(O)CH2CO2H −O2CC(O)CH2CO2H + H+, pKa = 2.22 :−O2CC(O)CH2CO2H −O2CC(O)CH2CO2− + H+, pKa = 3.89 At high pH, the enolizable proton is ionized: :−O2CC(O)CH2CO2− −O2CC(O−)CHCO2− + H+, pKa = 13.03 The enol forms of oxaloacetic acid are particularly stable. Keto-enol tautomerization is catalyzed by the enzyme oxaloacetate tautomerase. ''trans''-Enol-oxaloacetate also appears when tartrate is the substrate for fumarase. Biosynthesis Oxaloacetate forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is a metabolic intermediate in the citric acid cycle. Etymology The word 'malic' is derived from Latin , meaning 'apple'. The related Latin word , meaning 'apple tree', is used as the name of the genus '' Malus'', which includes all apples and crabapples; and is the origin of other taxonomic classifications such as Maloideae, Malinae, and Maleae. Biochemistry L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically. File:L-Äpfelsäure.svg, L-Malic acid (''S'') File:D-Äpfelsäure.svg, D-Malic acid (''R'') Malate plays an important role ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumaric Acid
Fumaric acid or ''trans''-butenedioic acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297. The salts and esters are known as fumarates. Fumarate can also refer to the ion (in solution). Fumaric acid is the ''trans'' isomer of butenedioic acid, while maleic acid is the ''cis'' isomer. Biosynthesis and occurrence It is produced in eukaryotic organisms from succinate in complex 2 of the electron transport chain via the enzyme succinate dehydrogenase. Fumaric acid is found in fumitory (''Fumaria officinalis''), bolete mushrooms (specifically ''Boletus fomentarius var. pseudo-igniarius''), lichen, and Iceland moss. Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food. It is formed by the oxidation of succinate by the enzyme succinate dehydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinate
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into Fumaric acid, fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making Adenosine triphosphate, ATP, and as a signaling molecule reflecting the cellular metabolic state. Succinate is generated in mitochondria via the citric acid cycle, tricarboxylic acid (TCA) cycle. Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling. As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore ATP synthesis, happens in some genetic m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A. Sources It is an important intermediate in the citric acid cycle, where it is synthesized from Alpha-Ketoglutaric acid, α-ketoglutarate by Alpha-ketoglutarate dehydrogenase, α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added. With B12 as an enzymatic cofactor, it is also synthesized from propionyl coenzymeA, propionyl CoA, the odd-numbered fatty acid, which cannot undergo beta-oxidation. Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12, vitamin B12-dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA. Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocitric Acid
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salts and esters of isocitric acid are known as isocitrates. The isocitrate anion is a substrate of the citric acid cycle. Isocitrate is formed from citrate with the help of the enzyme aconitase, and is acted upon by isocitrate dehydrogenase. Isocitric acid is commonly used as a marker to detect the authenticity and quality of fruit products, most often citrus juices. In authentic orange juice, for example, the ratio of citric acid to D-isocitric acid is usually less than 130. An isocitric acid value higher than this may be indicative of fruit juice adulteration. Isocitric acid has largely been used as a biochemical agent due to limited amounts. However, isocitric acid has been shown to have pharmaceutical and therapeutic effects. Isocitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aconitic Acid
Aconitic acid refers to organic compounds with the formula . A white solid, it is classified as a tricarboxylic acid. The two isomers are ''cis''-aconitic acid and ''trans''-aconitic acid. The conjugate base of ''cis''-aconitic acid, ''cis''-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase. Aconitic acid can be synthesized by dehydration of citric acid using sulfuric acid: :(HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O A mixture of isomers is generated in this way. Aconitic acid was originally isolated from ''Aconitum napellus'' by Swiss chemist and apothecary Jacques Peschier in 1820. It was first prepared by thermal dehydration. Like the conjugate bases of other polycarboxylic acid, acotinic acid forms a variety of coordination complexes. One example is the coordination polymer Coordination may refer to: * Coordination (linguistics), a compound grammatical construction * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |