|
Π-interactions
In chemistry, ¤Ç-effects or ¤Ç-interactions are a type of non-covalent interaction that involves Pi bond, ¤Ç systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich ¤Ç system can interact with a metal (cationic or neutral), an anion, another molecule and even another ¤Ç system. Non-covalent interactions involving ¤Ç systems are pivotal to biological events such as protein-ligand recognition. Types The most common types of ¤Ç-interactions involve: *MetalÔÇô¤Ç interactions: involves interaction of a metal and the face of a ¤Ç system, the metal can be a cation (known as CationÔÇôpi interaction, cationÔÇô¤Ç interactions) or neutral *PolarÔÇô¤Ç interactions: involves interaction of a polar molecule and quadrupole moment a ¤Ç system. *Pi stacking, AromaticÔÇôaromatic interactions (¤Ç stacking): involves interactions of aromatic molecules with each other. **AreneÔÇôperfluoroarene interaction: electron-rich ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Bond
In chemistry, pi bonds (¤Ç bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. The Greek letter ¤Ç in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. This latter mode forms part of the basis for metal-metal multiple bonding. Properties Pi bonds are usually weaker than sigma bonds. The CÔÇôC double bond, composed of one sigma and o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Honeycomb Lattice
The hexagonal lattice (sometimes called triangular lattice) is one of the five two-dimensional Bravais lattice types. The symmetry category of the lattice is wallpaper group p6m. The primitive translation vectors of the hexagonal lattice form an angle of 120┬░ and are of equal lengths, : , \mathbf a_1, = , \mathbf a_2, = a. The reciprocal lattice of the hexagonal lattice is a hexagonal lattice in reciprocal space with orientation changed by 90┬░ and primitive lattice vectors of length : g=\frac. Honeycomb point set The honeycomb point set is a special case of the hexagonal lattice with a two-atom basis. The centers of the hexagons of a honeycomb form a hexagonal lattice, and the honeycomb point set can be seen as the union of two offset hexagonal lattices. In nature, carbon atoms of the two-dimensional material graphene are arranged in a honeycomb point set. Crystal classes The ''hexagonal lattice'' class names, Sch├Ânflies notation, Hermann-Mauguin notation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid State Communications
Solid State Communications is a peer-reviewed scientific journal of solid-state physics. The journal specializes in short papers on significant developments in the condensed matter science. The journal was established 1963, when the ''Journal of Physics and Chemistry of Solids'' split its letters section to create this journal. Elias Burstein served as founding chief editor until 1992, and was succeeded by Manuel Cardona until 2004, when Aron Pinczuk assumed the role. Pinczuk stepped down in 2020. The journal is published bimonthly by Elsevier and its current editor-in-chief is François Peeters (University of Antwerp). Abstracting and Indexing The journal is abstracted and indexing in the following databases: *Cambridge Scientific Abstracts *Chemical Abstracts *Current Contents/Physics, Chemical, & Earth Sciences *Current Contents/ SciSearch Database *Current Contents/Social & Behavioral Sciences *MSCI *Engineering Index *INSPEC * PASCAL/CNRS *Research Alert * SSSA/ CISA/ EC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrathiafulvalene
Tetrathiafulvalene (TTF) is an organosulfur compound with the formula . It is the parent of many tetrathiafulvenes. Studies on these heterocyclic compound contributed to the development of molecular electronics, although no practical applications of TTF emerged. TTF is related to the hydrocarbon fulvalene () by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives. Preparation The high level of interest in TTFs spawned many syntheses of TTF and its analogues. Most preparations entail the coupling of cyclic building blocks such as 1,3-dithiole-2-thion or the related 1,3-dithiole-2-ones. For TTF itself, the synthesis begins with the cyclic trithiocarbonate ( 1,3-dithiole-2-thione), which is ''S''-methylated and then reduced to give (1,3-dithiole-2-yl methyl thioether), which is treated as follows: Protonolysis of a thioether: : Followed by deprotonation of the dithiolium cation with triethylamine: : Redox prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TCNQ
Tetracyanoquinodimethane (TCNQ) is an organic compound with the chemical formula . It is an orange crystalline solid. This cyanocarbon, a relative of para-quinone, is an electron acceptor that is used to prepare charge transfer salts, which are of interest in molecular electronics. Preparation and structure TCNQ is prepared by the condensation of 1,4-cyclohexanedione with malononitrile, followed by dehydrogenation of the resulting diene with bromine: : : The molecule is planar, with D2h symmetry. Reactions Like tetracyanoethylene (TCNE), TCNQ is easily reduced to give a blue-coloured radical anion. The reduction potential is about 0.3 V relative to the ferrocene/ ferrocenium couple. This property is exploited in the development of charge-transfer salts. TCNQ also forms complexes with electron-rich metal complexes. Charge transfer salts TCNQ achieved great attention because it forms charge-transfer salts with high electrical conductivity. These discoveries were influe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology " mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Assembly
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, piÔÇôpi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, molecular folding, molecular recognition, hostÔÇôguest chemistry, mechanically-interlocked molecular architectures, and dynamic covalent chemistry. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Recognition
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatics, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, coordination complex, metal coordination, hydrophobic effect, hydrophobic forces, van der Waals forces, piÔÇôpi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, folding (chemistry), molecular folding, molecular recognition, hostÔÇôgues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
24 Fig
4 (four) is a number, numeral and digit. It is the natural number following 3 and preceding 5. It is a square number, the smallest semiprime and composite number, and is considered unlucky in many East Asian cultures. Evolution of the Hindu-Arabic digit Brahmic numerals represented 1, 2, and 3 with as many lines. 4 was simplified by joining its four lines into a cross that looks like the modern plus sign. The Shunga would add a horizontal line on top of the digit, and the Kshatrapa and Pallava evolved the digit to a point where the speed of writing was a secondary concern. The Arabs' 4 still had the early concept of the cross, but for the sake of efficiency, was made in one stroke by connecting the "western" end to the "northern" end; the "eastern" end was finished off with a curve. The Europeans dropped the finishing curve and gradually made the digit less cursive, ending up with a digit very close to the original Brahmin cross. While the shape of the character for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alzheimer's Disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60ÔÇô70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation (including easily getting lost), mood swings, loss of motivation, self-neglect, and behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the average life expectancy following diagnosis is three to twelve years. The causes of Alzheimer's disease remain poorly understood. There are many environmental and genetic risk factors associated with its development. The strongest genetic risk factor is from an allele of apolipoprotein E. Other risk factors include a history of head injury, clinical depression, and high blood pressure. The progression of the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tacrine
Tacrine is a centrally acting acetylcholinesterase inhibitor and indirect cholinergic agonist (parasympathomimetic). It was the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer's disease, and was marketed under the trade name Cognex. Tacrine was first synthesised by Adrien Albert at the University of Sydney in 1949. It also acts as a histamine N-methyltransferase inhibitor. Clinical use Tacrine was the prototypical cholinesterase inhibitor for the treatment of Alzheimer's disease. William K. Summers received a patent for this use in 1989. Studies found that it may have a small beneficial effect on cognition and other clinical measures, though study data was limited and the clinical relevance of these findings was unclear.. Tacrine has been discontinued in the US in 2013, due to concerns over safety. Tacrine was also described as an analeptic agent used to promote mental alertness. Adverse effects ;Very common (>10% incidence) adverse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
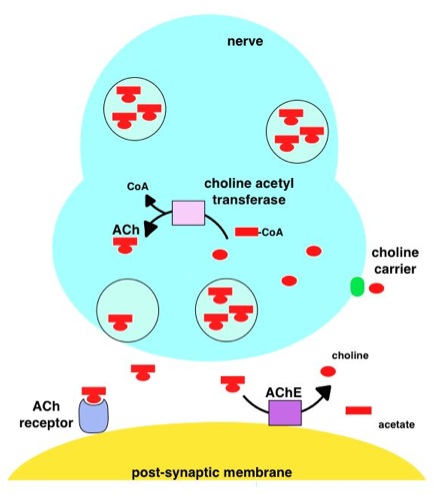
Acetylcholinesterase
Acetylcholinesterase (HUGO Gene Nomenclature Committee, HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalysis, catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate cholinergic neurotransmission, synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activityÔÇöeach molecule of AChE degrades about 5,000 molecules of acetylcholine (ACh) per second, approaching the limit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |