Vibronic spectroscopy on:
[Wikipedia]
[Google]
[Amazon]
Vibronic spectroscopy is a branch of molecular
 The treatment of rotational fine structure of vibronic transitions is similar to the treatment of rotation-vibration transitions and differs principally in the fact that the ground and excited states correspond to two different electronic states as well as to two different vibrational levels. For the P-branch , so that
:
::
Similarly for the R-branch , and
:
::
Thus, the wavenumbers of transitions in both P- and R- branches are given, to a first approximation, by the single formula
:
Here positive ''m'' values refer to the R-branch (with ''m'' = +''J'' ′ = ''J
The treatment of rotational fine structure of vibronic transitions is similar to the treatment of rotation-vibration transitions and differs principally in the fact that the ground and excited states correspond to two different electronic states as well as to two different vibrational levels. For the P-branch , so that
:
::
Similarly for the R-branch , and
:
::
Thus, the wavenumbers of transitions in both P- and R- branches are given, to a first approximation, by the single formula
:
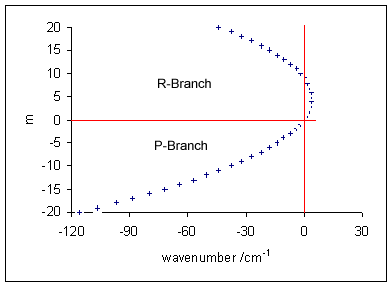
Here positive ''m'' values refer to the R-branch (with ''m'' = +''J'' ′ = ''J'' +1'') and negative values refer to the P-branch (with ''m'' = -''J'' ′′). The wavenumbers of the lines in the P-branch, on the low wavenumber side of the ''band origin'' at , increase with ''m''. In the R-branch, for the usual case that ''B''′ < ''B''′′, as ''J'' increases the wavenumbers at first lie increasingly on the high wavenumber side of the band origin but then start to decrease, eventually lying on the low wavenumber side. The Fortrat diagram illustrates this effect.When centrifugal distortion is included the R-branch lines below the vibrational origin do not coincide with P-branch lines In the rigid rotor approximation the line wavenumbers lie on a
 As an example from
As an example from
spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter ...
concerned with vibronic transitions: the simultaneous changes in electronic
Electronic may refer to:
*Electronics, the science of how to control electric energy in semiconductor
* ''Electronics'' (magazine), a defunct American trade journal
*Electronic storage, the storage of data using an electronic device
*Electronic co ...
and vibrational energy levels of a molecule due to the absorption or emission of a photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they alwa ...
of the appropriate energy. In the gas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetiza ...
, vibronic transitions are accompanied by changes in rotational energy also.
Vibronic spectra of diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. O ...
s have been analysed in detail; emission spectra are more complicated than absorption spectra. The intensity of allowed vibronic transitions is governed by the Franck–Condon principle. Vibronic spectroscopy may provide information, such as bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
, on electronic excited states of stable molecules. It has also been applied to the study of unstable
In numerous fields of study, the component of instability within a system is generally characterized by some of the outputs or internal states growing without bounds. Not all systems that are not stable are unstable; systems can also be mar ...
molecules such as dicarbon, C2, in discharges, flames
A flame (from Latin '' flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they ...
and astronomical objects.Hollas, p. 211.
Principles
Electronic transitions are typically observed in the visible and ultraviolet regions, in the wavelength range approximately 200–700 nm (50,000–14,000 cm−1), whereas fundamental vibrations are observed below about 4000 cm−1.Energy is related to wavenumber by , where h= Planck's constant and c is the velocity of light When the electronic and vibrational energy changes are so different,vibronic coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" ...
(mixing of electronic and vibrational wave function
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements ...
s) can be neglected and the energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
of a vibronic level can be taken as the sum of the electronic and vibrational (and rotational) energies; that is, the Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and elect ...
applies. The overall molecular energy depends not only on the electronic state but also on vibrational and rotational quantum numbers, denoted v and J respectively for diatomic molecules. It is conventional to add a double prime (v", J") for levels of the electronic ground state and a single prime (v', J') for electronically excited states.
Each electronic transition may show vibrational coarse structure, and for molecules in the gas phase, rotational fine structure. This is true even when the molecule has a zero dipole moment and therefore has no vibration-rotation infrared spectrum or pure rotational microwave spectrum.
It is necessary to distinguish between absorption and emission spectra. With absorption the molecule starts in the ground electronic state, and usually also in the vibrational ground state because at ordinary temperatures the energy necessary for vibrational excitation is large compared to the average thermal energy. The molecule is excited to another electronic state and to many possible vibrational states . With emission, the molecule can start in various populated vibrational states, and finishes in the electronic ground state in one of many populated vibrational levels. The emission spectrum is more complicated than the absorption spectrum of the same molecule because there are more changes in vibrational energy level.
For absorption spectra, the vibrational coarse structure for a given electronic transition forms a single ''progression'', or series of transitions with a common level, here the lower level . There are no selection rules for vibrational quantum numbers, which are zero in the ground vibrational level of the initial electronic ground state, but can take any integer values in the final electronic excited state. The term values for a harmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its equilibrium position, experiences a restoring force ''F'' proportional to the displacement ''x'':
\vec F = -k \vec x,
where ''k'' is a positive const ...
are given by
:
where ''v'' is a vibrational quantum number, ωe is the harmonic wavenumber. In the next approximation the term values are given by
:
where χe is an anharmonicity
In classical mechanics, anharmonicity is the deviation of a system from being a harmonic oscillator. An oscillator that is not oscillating in harmonic motion is known as an anharmonic oscillator where the system can be approximated to a harmo ...
constant. This is, in effect, a better approximation to the Morse potential
The Morse potential, named after physicist Philip M. Morse, is a convenient
interatomic interaction model for the potential energy of a diatomic molecule. It is a better approximation for the vibrational structure of the molecule than the qua ...
near the potential minimum. The spacing between adjacent vibrational lines decreases with increasing quantum number because of anharmonicity in the vibration. Eventually the separation decreases to zero when the molecule photo-dissociates into a continuum of states. The second formula is adequate for small values of the vibrational quantum number. For higher values further anharmonicity terms are needed as the molecule approaches the dissociation limit, at the energy corresponding to the upper (final state) potential curve at infinite internuclear distance.
The intensity of allowed vibronic transitions is governed by the Franck–Condon principle. Since electronic transitions are very fast compared with nuclear motions, vibrational levels are favored when they correspond to a minimal change in the nuclear coordinates, that is, when the transition is "vertical" on the energy level diagram. Each line has a finite linewidth
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to ident ...
, dependent on a variety of factors.
Vibronic spectra of diatomic molecules in the gas phase have been analyzed in detail. Vibrational coarse structure can sometimes be observed in the spectra of molecules in liquid or solid phases and of molecules in solution. Related phenomena including photoelectron spectroscopy, resonance Raman spectroscopy, luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crys ...
, and fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
are not discussed in this article, though they also involve vibronic transitions.
Diatomic molecules
The vibronic spectra of diatomic molecules in the gas phase also show rotational fine structure. Each line in a vibrational progression will show P- and R- branches. For some electronic transitions there will also be a Q-branch. The transition energies, expressed in wavenumbers, of the lines for a particular vibronic transition are given, in therigid rotor
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special ri ...
approximation, that is, ignoring centrifugal distortion, byBanwell and McCash, p. 171
:
Here ''B'' are rotational constant
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special rig ...
s and ''J'' are rotational quantum number
In quantum physics and chemistry, quantum numbers describe values of conserved quantities in the dynamics of a quantum system. Quantum numbers correspond to eigenvalues of operators that commute with the Hamiltonian—quantities that can ...
s. (For B also, a double prime indicates the ground state and a single prime an electronically excited state.) The values of the rotational constants may differ appreciably because the bond length in the electronic excited state may be quite different from the bond length in the ground state, because of the operation of the Franck-Condon principle. The rotational constant is inversely proportional to the square of the bond length. Usually ''B''′ < ''B''′′ as is true when an electron is promoted from a bonding orbital to an antibonding orbital
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more n ...
, causing bond lengthening. But this is not always the case; if an electron is promoted from a non-bonding or antibonding orbital to a bonding orbital, there will be bond-shortening and ''B''′ > ''B''′′.
 The treatment of rotational fine structure of vibronic transitions is similar to the treatment of rotation-vibration transitions and differs principally in the fact that the ground and excited states correspond to two different electronic states as well as to two different vibrational levels. For the P-branch , so that
:
::
Similarly for the R-branch , and
:
::
Thus, the wavenumbers of transitions in both P- and R- branches are given, to a first approximation, by the single formula
:
Here positive ''m'' values refer to the R-branch (with ''m'' = +''J'' ′ = ''J
The treatment of rotational fine structure of vibronic transitions is similar to the treatment of rotation-vibration transitions and differs principally in the fact that the ground and excited states correspond to two different electronic states as well as to two different vibrational levels. For the P-branch , so that
:
::
Similarly for the R-branch , and
:
::
Thus, the wavenumbers of transitions in both P- and R- branches are given, to a first approximation, by the single formula
:
Here positive ''m'' values refer to the R-branch (with ''m'' = +''J'' ′ = ''Jparabola
In mathematics, a parabola is a plane curve which is mirror-symmetrical and is approximately U-shaped. It fits several superficially different mathematical descriptions, which can all be proved to define exactly the same curves.
One descri ...
which has a maximum at
:
The line of highest wavenumber in the R-branch is known as the ''band head''. It occurs at the value of ''m'' which is equal to the integer part of ''x'', or of (''x''+1).
When a Q- branch is allowed for a particular electronic transition, the lines of the Q-branch correspond to the case ∆''J''=0, ''J''′=''J′′'' and wavenumbers are given by
:
The Q-branch then consists of a series of lines with increasing separation between adjacent lines as ''J'' increases. When ''B''′<''B''′′ the Q-branch lies to lower wavenumbers relative to the vibrational line.
Predissociation
The phenomenon of predissociation occurs when an electronic transition results in dissociation of the molecule at an excitation energy less than the normal dissociation limit of the upper state. This can occur when the potential energy curve of the upper state crosses the curve for a repulsive state, so that the two states have equal energy at some internuclear distance. This allows the possibility of a radiationless transition to the repulsive state whose energy levels form a continuum, so that there is blurring of the particular vibrational band in the vibrational progression.Applications
The analysis of vibronic spectra of diatomic molecules provides information concerning both the ground electronic state and the excited electronic state. Data for the ground state can also be obtained by vibrational or pure rotational spectroscopy, but data for the excited state can only be obtained from the analysis of vibronic spectra. For example, the bond length in the excited state may be derived from the value of the rotational constant ''B''′. In addition to stable diatomic molecules, vibronic spectroscopy has been used to study unstable species, including CH, NH,hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
, OH, and cyano radical, CN. The Swan bands in hydrocarbon flame spectra are a progression in the C-C stretching vibration of the dicarbon radical, C2 for the electronic transition. Vibronic bands for 9 other electronic transitions of C2 have been observed in the infrared and ultraviolet regions.
Polyatomic molecules and ions
For polyatomic molecules, progressions are most often observed when the change in bond lengths upon electronic excitation coincides with the change due to a ″totally symmetric″ vibration.In a ″totally symmetric″ vibration the lengths of all symmetrically-equivalent bonds vary in phase with each other. The symmetry of the molecule is the same in the vibrational excited state as in the vibrational ground state. This is the same process that occurs in resonance Raman spectroscopy. For example, informaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
(methanal), H2CO, the n → π* transition involves excitation of an electron from a non-bonding orbital to an antibonding pi orbital which weakens and lengthens the C-O bond. This produces a long progression in the C-O stretching vibration. Another example is furnished by benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, C6H6. In both gas and liquid phase the band around 250 nm shows a progression in the symmetric ring-breathing vibration.
 As an example from
As an example from inorganic chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disci ...
the permanganate
A permanganate () is a chemical compound containing the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a ...
ion, , in aqueous solution has an intense purple colour due to an O → Mn ligand-to-metal charge transfer band
193px, The intense color of tris(bipyridine)ruthenium(II) arises from a metal-to-ligand charge-transfer band.
Charge-transfer bands are a characteristic feature of the optical spectra of many compounds. These bands are typically more intense tha ...
(LMCT) in much of the visible region. This band shows a progression in the symmetric Mn-O stretching vibration. The individual lines overlap each other extensively, giving rise to a broad overall profile with some coarse structure.
Progressions in vibrations which are not totally symmetric may also be observed.
''d-d'' electronic transitions in atoms in a centrosymmetric
In crystallography, a centrosymmetric point group contains an inversion center as one of its symmetry elements. In such a point group, for every point (x, y, z) in the unit cell there is an indistinguishable point (-x, -y, -z). Such point gr ...
environment are electric-dipole forbidden by the Laporte rule. This will apply to octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet a ...
coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Man ...
s of the transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s. The spectra of many of these complexes have some vibronic character. The same rule also applies to ''f-f'' transitions in centrosymmetric complexes of lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
s and actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s. In the case of the octahedral actinide chloro-complex of uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
(IV), UCl62− the observed electronic spectrum is entirely vibronic. At the temperature of liquid helium, 4K, the vibronic structure was completely resolved, with zero intensity for the purely electronic transition, and three side-lines corresponding to the asymmetric U-Cl stretching vibration and two asymmetric Cl-U-Cl bending modes. Later studies on the same anion were also able to account for vibronic transitions involving low-frequency lattice vibrations.
Notes
References
Bibliography
* Chapter: Molecular Spectroscopy 2. * * * * {{Branches of spectroscopy Spectroscopy