Valley of stability on:
[Wikipedia]
[Google]
[Amazon]
In
File:BindingNuDat2.png, Chart of nuclides (isotopes) by binding energy, depicting the valley of stability. The diagonal line corresponds to equal numbers of neutrons and protons. Dark blue squares represent nuclides with the greatest binding energy, hence they correspond to the most stable nuclides. The binding energy is greatest along the floor of the valley of stability.
File:HalflifeNuDat2.png, Chart of nuclides by half life. Black squares represent nuclides with the longest half lives hence they correspond to the most stable nuclides. The most stable, long-lived nuclides lie along the floor of the valley of stability. Nuclides with more than 20 protons must have more neutrons than protons to be stable.
File:DecayModeNuDat2.png, Chart of nuclides by type of decay. Black squares are stable nuclides. Nuclides with excessive neutrons or protons are unstable to β− (light blue) or β+ (green) decay, respectively. At high atomic number, alpha emission (orange) or spontaneous fission (dark blue) become common decay modes.
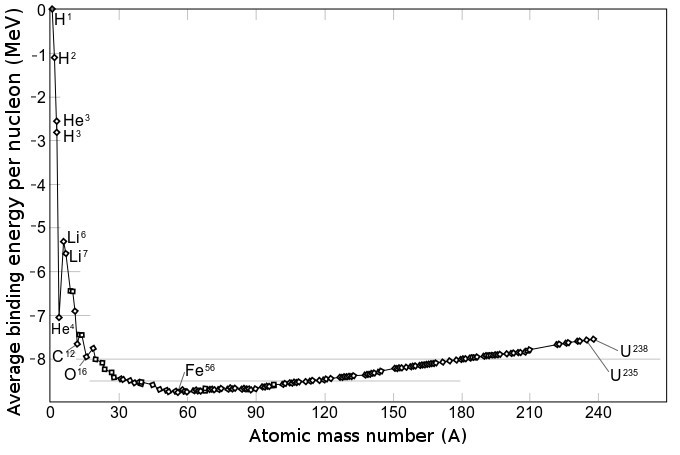 The figure at right shows the average binding energy per nucleon as a function of atomic mass number along the line of beta stability, that is, along the bottom of the valley of stability. For very small atomic mass number (H, He, Li), binding energy per nucleon is small, and this energy increases rapidly with atomic mass number. Nickel-62 (28 protons, 34 neutrons) has the highest mean binding energy of all nuclides, while iron-58 (26 protons, 32 neutrons) and
The figure at right shows the average binding energy per nucleon as a function of atomic mass number along the line of beta stability, that is, along the bottom of the valley of stability. For very small atomic mass number (H, He, Li), binding energy per nucleon is small, and this energy increases rapidly with atomic mass number. Nickel-62 (28 protons, 34 neutrons) has the highest mean binding energy of all nuclides, while iron-58 (26 protons, 32 neutrons) and  The figure at right shows the average binding energy per nucleon across the valley of stability for nuclides with mass number ''A'' = 125. At the bottom of this curve is
The figure at right shows the average binding energy per nucleon across the valley of stability for nuclides with mass number ''A'' = 125. At the bottom of this curve is
 Radioactive decay often proceeds via a sequence of steps known as a decay chain. For example, 238U decays to 234Th which decays to 234mPa and so on, eventually reaching 206Pb:
:
With each step of this sequence of reactions, energy is released and the
Radioactive decay often proceeds via a sequence of steps known as a decay chain. For example, 238U decays to 234Th which decays to 234mPa and so on, eventually reaching 206Pb:
:
With each step of this sequence of reactions, energy is released and the  The fission processes that occur within
The fission processes that occur within
The Live Chart of Nuclides - IAEA
with filter on decay type
The Valley of Stability (video)
– a virtual "flight" through 3D representation of the nuclide chart, by CEA (France)
The nuclear landscape: The variety and abundance of nuclei
– Chapter 6 of the book ''Nucleus: A trip into the heart of matter'' by Mackintosh, Ai-Khalili, Jonson, and Pena describes the valley of stability and its implications (Baltimore, Maryland:The Johns Hopkins University Press), 2001. {{ISBN, 0-801 8-6860-2 Nuclear physics Radioactivity Isotopes
nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies th ...
, the valley of stability (also called the belt of stability, nuclear valley, energy valley, or beta stability valley) is a characterization of the stability of nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
s to radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
based on their binding energy. Nuclides are composed of proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s. The shape of the valley refers to the profile of binding energy as a function of the numbers of neutrons and protons, with the lowest part of the valley corresponding to the region of most stable nuclei. The line of stable nuclides down the center of the valley of stability is known as the line of beta stability. The sides of the valley correspond to increasing instability to beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
(β− or β+). The decay of a nuclide becomes more energetically favorable the further it is from the line of beta stability. The boundaries of the valley correspond to the nuclear drip line
The nuclear drip line is the boundary beyond which atomic nuclei are unbound with respect to the emission of a proton or neutron.
An arbitrary combination of protons and neutrons does not necessarily yield a stable atomic nucleus, nucleus. One ...
s, where nuclides become so unstable they emit single protons or single neutrons. Regions of instability within the valley at high atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
also include radioactive decay by alpha radiation
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an atom ...
or spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
. The shape of the valley is roughly an elongated paraboloid
In geometry, a paraboloid is a quadric surface that has exactly one axial symmetry, axis of symmetry and no central symmetry, center of symmetry. The term "paraboloid" is derived from parabola, which refers to a conic section that has a similar p ...
corresponding to the nuclide binding energies as a function of neutron and atomic numbers.
The nuclides within the valley of stability encompass the entire table of nuclides. The chart of those nuclides is also known as a Segrè chart, after the physicist Emilio Segrè. The Segrè chart may be considered a map of the nuclear valley. The region of proton and neutron combinations outside of the valley of stability is referred to as the sea of instability.
Scientists have long searched for long-lived heavy isotopes outside of the valley of stability, hypothesized by Glenn T. Seaborg in the late 1960s. These relatively stable nuclides are expected to have particular configurations of " magic" atomic and neutron numbers, and form a so-called island of stability.
Description
All atomic nuclei are composed of protons and neutrons bound together by thenuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both ...
. There are 286 primordial nuclides that occur naturally on earth, each corresponding to a unique number of protons, called the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
, ''Z'', and a unique number of neutrons, called the neutron number, ''N''. The mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
, ''A'', of a nuclide is the sum of atomic and neutron numbers, ''A'' = ''Z'' + ''N''. Not all nuclides are stable, however. According to Byrne, stable nuclides are defined as those having a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
greater than 1018 years, and there are many combinations of protons and neutrons that form nuclides that are unstable. A common example of an unstable nuclide is carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and coll ...
that decays by beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
into nitrogen-14
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Thirteen radioisotopes are also known, with atomic masses ranging from 9 to 23, ...
with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of about 5,730 years:
: → + +
In this form of decay, the original element becomes a new chemical element in a process known as nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
A transmutat ...
and a beta particle and an electron antineutrino
A neutrino ( ; denoted by the Greek letter ) is an elementary particle that interacts via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small ('' -ino'') that it ...
are emitted. An essential property of this and all nuclide decays is that the total energy of the decay product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( d ...
is less than that of the original nuclide. The difference between the initial and final nuclide binding energies is carried away by the kinetic energies of the decay products, often the beta particle and its associated neutrino.
The concept of the valley of stability is a way of organizing all of the nuclides according to binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
as a function of neutron and proton numbers. Most stable nuclides have roughly equal numbers of protons and neutrons, so the line for which ''Z'' = ''N'' forms a rough initial line defining stable nuclides. The greater the number of protons, the more neutrons are required to stabilize a nuclide; nuclides with larger values for ''Z'' require an even larger number of neutrons, ''N'' > ''Z'', to be stable. The valley of stability is formed by the negative of binding energy, the binding energy being the energy required to break apart the nuclide into its proton and neutron components. The stable nuclides have high binding energy, and these nuclides lie along the bottom of the valley of stability. Nuclides with weaker binding energy have combinations of ''N'' and ''Z'' that lie off of the line of stability and further up the sides of the valley of stability. Unstable nuclides can be formed in nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction. They are used for commercial electricity, marine propulsion, weapons production and research. Fissile nuclei (primarily uranium-235 or plutonium-2 ...
or supernovas, for example. Such nuclides often decay in sequences of reactions called decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
s that take the resulting nuclides sequentially down the slopes of the valley of stability. The sequence of decays take nuclides toward greater binding energies, and the nuclides terminating the chain are stable. The valley of stability provides both a conceptual approach for how to organize the myriad stable and unstable nuclides into a coherent picture and an intuitive way to understand how and why sequences of radioactive decay occur.
The role of neutrons
The protons and neutrons that comprise an atomic nucleus behave almost identically within the nucleus. The approximate symmetry ofisospin
In nuclear physics and particle physics, isospin (''I'') is a quantum number related to the up- and down quark content of the particle.
Isospin is also known as isobaric spin or isotopic spin.
Isospin symmetry is a subset of the flavour symmetr ...
treats these particles as identical, but in a different quantum state. This symmetry is only approximate, however, and the nuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both ...
that binds nucleons together is a complicated function depending on nucleon type, spin state, electric charge, momentum, etc. and with contributions from non- central forces. The nuclear force is not a fundamental force of nature, but a consequence of the residual effects of the strong force that surround the nucleons. One consequence of these complications is that although deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
, a bound state of a proton (p) and a neutron (n) is stable, exotic nuclides such as diproton
Helium (He) ( standard atomic weight: ) has nine known isotopes, but only helium-3 (He) and helium-4 (He) are stable. All radioisotopes are short-lived; the longest-lived is He with half-life . The least stable is He, with half-life (), though He ...
or dineutron are unbound. The nuclear force is not sufficiently strong to form either p-p or n-n bound states, or equivalently, the nuclear force does not form a potential well
A potential well is the region surrounding a local minimum of potential energy. Energy captured in a potential well is unable to convert to another type of energy ( kinetic energy in the case of a gravitational potential well) because it is cap ...
deep enough to bind these identical nucleons.
Stable nuclides require approximately equal numbers of protons and neutrons. The stable nuclide carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon ( carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-1 ...
(12C) is composed of six neutrons and six protons, for example. Protons have a positive charge, hence within a nuclide with many protons there are large repulsive forces between protons arising from the Coulomb force. By acting to separate protons from one another, the neutrons within a nuclide play an essential role in stabilizing nuclides. With increasing atomic number, even greater numbers of neutrons are required to obtain stability. The heaviest stable element, lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
(Pb), has many more neutrons than protons. The stable nuclide 206Pb has ''Z'' = 82 and ''N'' = 124, for example. For this reason, the valley of stability does not follow the line ''Z'' = ''N'' for A larger than 40 (''Z'' = 20 is the element calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
). Neutron number increases along the line of beta stability at a faster rate than atomic number.
The line of beta stability follows a particular curve of neutron–proton ratio
The neutron–proton ratio (N/Z ratio or nuclear ratio) of an atomic nucleus is the ratio of its number of neutrons to its number of protons. Among stable nuclei and naturally occurring nuclei, this ratio generally increases with increasing atomi ...
, corresponding to the most stable nuclides. On one side of the valley of stability, this ratio is small, corresponding to an excess of protons over neutrons in the nuclides. These nuclides tend to be unstable to β+ decay or electron capture, since such decay converts a proton to a neutron. The decay serves to move the nuclides toward a more stable neutron-proton ratio. On the other side of the valley of stability, this ratio is large, corresponding to an excess of neutrons over protons in the nuclides. These nuclides tend to be unstable to β− decay, since such decay converts neutrons to protons. On this side of the valley of stability, β− decay also serves to move nuclides toward a more stable neutron-proton ratio.
Neutrons, protons, and binding energy
The mass of an atomic nucleus is given by : where and are the rest mass of a proton and a neutron, respectively, and is the totalbinding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
of the nucleus. The mass–energy equivalence
In physics, mass–energy equivalence is the relationship between mass and energy in a system's rest frame. The two differ only by a multiplicative constant and the units of measurement. The principle is described by the physicist Albert Einstei ...
is used here. The binding energy is subtracted from the sum of the proton and neutron masses because the mass of the nucleus is ''less'' than that sum. This property, called the mass defect, is necessary for a stable nucleus; within a nucleus, the nuclides are trapped by a potential well
A potential well is the region surrounding a local minimum of potential energy. Energy captured in a potential well is unable to convert to another type of energy ( kinetic energy in the case of a gravitational potential well) because it is cap ...
. A semi-empirical mass formula states that the binding energy will take the form
:
The difference between the mass of a nucleus and the sum of the masses of the neutrons and protons that comprise it is known as the mass defect. EB is often divided by the mass number to obtain binding energy per nucleon for comparisons of binding energies between nuclides. Each of the terms in this formula has a theoretical basis. The coefficients , , , and a coefficient that appears in the formula for are determined empirically.
The binding energy expression gives a quantitative estimate for the neutron-proton ratio. The energy is a quadratic expression in that is minimized when the neutron-proton ratio is . This equation for the neutron-proton ratio shows that in stable nuclides the number of neutrons is greater than the number of protons by a factor that scales as .
 The figure at right shows the average binding energy per nucleon as a function of atomic mass number along the line of beta stability, that is, along the bottom of the valley of stability. For very small atomic mass number (H, He, Li), binding energy per nucleon is small, and this energy increases rapidly with atomic mass number. Nickel-62 (28 protons, 34 neutrons) has the highest mean binding energy of all nuclides, while iron-58 (26 protons, 32 neutrons) and
The figure at right shows the average binding energy per nucleon as a function of atomic mass number along the line of beta stability, that is, along the bottom of the valley of stability. For very small atomic mass number (H, He, Li), binding energy per nucleon is small, and this energy increases rapidly with atomic mass number. Nickel-62 (28 protons, 34 neutrons) has the highest mean binding energy of all nuclides, while iron-58 (26 protons, 32 neutrons) and iron-56
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56.
Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei.
...
(26 protons, 30 neutrons) are a close second and third. These nuclides lie at the very bottom of the valley of stability. From this bottom, the average binding energy per nucleon slowly decreases with increasing atomic mass number. The heavy nuclide 238U is not stable, but is slow to decay with a half-life of 4.5 billion years. It has relatively small binding energy per nucleon.
For β− decay, nuclear reactions have the generic form
: → + +
where and are the mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
of the decaying nucleus, and X and X′ are the initial and final nuclides, respectively. For β+ decay, the generic form is
: → + +
These reactions correspond to the decay of a neutron to a proton, or the decay of a proton to a neutron, within the nucleus, respectively. These reactions begin on one side or the other of the valley of stability, and the directions of the reactions are to move the initial nuclides down the valley walls towards a region of greater stability, that is, toward greater binding energy.
 The figure at right shows the average binding energy per nucleon across the valley of stability for nuclides with mass number ''A'' = 125. At the bottom of this curve is
The figure at right shows the average binding energy per nucleon across the valley of stability for nuclides with mass number ''A'' = 125. At the bottom of this curve is tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
(52Te), which is stable. Nuclides to the left of 52Te are unstable with an excess of neutrons, while those on the right are unstable with an excess of protons. A nuclide on the left therefore undergoes β− decay, which converts a neutron to a proton, hence shifts the nuclide to the right and toward greater stability. A nuclide on the right similarly undergoes β+ decay, which shifts the nuclide to the left and toward greater stability.
Heavy nuclides are susceptible to α decay, and these nuclear reactions have the generic form,
: → +
As in β decay, the decay product X′ has greater binding energy and it is closer to the middle of the valley of stability. The α particle carries away two neutrons and two protons, leaving a lighter nuclide. Since heavy nuclides have many more neutrons than protons, α decay increases a nuclide's neutron-proton ratio.
Proton and neutron drip lines
The boundaries of the valley of stability, that is, the upper limits of the valley walls, are the neutron drip line on the neutron-rich side, and the proton drip line on the proton-rich side. The nucleon drip lines are at the extremes of the neutron-proton ratio. At neutron–proton ratios beyond the drip lines, no nuclei can exist. The location of the neutron drip line is not well known for most of the Segrè chart, whereas the proton and alpha drip lines have been measured for a wide range of elements. Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The difference in binding energy between neighboring nuclides increases as the sides of the valley of stability are ascended, and correspondingly the nuclide half-lives decrease, as indicated in the figure above. If one were to add nucleons one at a time to a given nuclide, the process will eventually lead to a newly formed nuclide that is so unstable that it promptly decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term "drip line". Proton emission is not seen in naturally occurring nuclides. Proton emitters can be produced vianuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s, usually utilizing linear particle accelerator
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of Oscillation, oscillating electric potentials along ...
s (linac). Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-151 and thulium-147 were observed at experiments at the GSI in West Germany. Research in the field flourished after this breakthrough, and to date more than 25 nuclides have been found to exhibit proton emission. The study of proton emission has aided the understanding of nuclear deformation, masses and structure, and it is an example of quantum tunneling
In physics, a quantum (: quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a property can be "quantized" is referred to as "the hypothesis of quantization". This me ...
.
Two examples of nuclides that emit neutrons are beryllium-13 (mean life ) and helium-5 (). Since only a neutron is lost in this process, the atom does not gain or lose any protons, and so it does not become an atom of a different element. Instead, the atom will become a new isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of the original element, such as beryllium-13 becoming beryllium-12 after emitting one of its neutrons.
In nuclear engineering
Nuclear engineering is the engineering discipline concerned with designing and applying systems that utilize the energy released by nuclear processes.
The most prominent application of nuclear engineering is the generation of electricity. Worldwide ...
, a prompt neutron is a neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
immediately emitted by a nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
event. Prompt neutrons emerge from the fission of an unstable fissionable or fissile
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal Nuclear chain reaction#Fission chain reaction, chain reaction can only be achieved with fissil ...
heavy nucleus almost instantaneously. Delayed neutron decay can occur within the same context, emitted after beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
of one of the fission products. Delayed neutron decay can occur at times from a few milliseconds to a few minutes. The U.S. Nuclear Regulatory Commission defines a prompt neutron as a neutron emerging from fission within 10−14 seconds.
Island of stability
The island of stability is a region outside the valley of stability where it is predicted that a set of heavyisotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
with near magic numbers of protons and neutrons will locally reverse the trend of decreasing stability in elements heavier than uranium.
The hypothesis for the island of stability is based upon the nuclear shell model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model utilizes the Pauli exclusion principle to model the structure of atomic nuclei in terms of energy levels. The first shell model was proposed by Dmitri Ivanenk ...
, which implies that the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
is built up in "shells" in a manner similar to the structure of the much larger electron shells in atoms. In both cases, shells are just groups of quantum energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
s that are relatively close to each other. Energy levels from quantum states in two different shells will be separated by a relatively large energy gap. So when the number of neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s and proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s completely fills the energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
s of a given shell in the nucleus, the binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
per nucleon will reach a local maximum and thus that particular configuration will have a longer lifetime than nearby isotopes that do not possess filled shells.
A filled shell would have " magic numbers" of neutrons and protons. One possible magic number of neutrons for spherical nuclei is 184, and some possible matching proton numbers are 114, 120 and 126. These configurations imply that the most stable spherical isotopes would be flerovium-298, unbinilium-304 and unbihexium-310. Of particular note is 298Fl, which would be " doubly magic" (both its proton number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
of 114 and neutron number of 184 are thought to be magic). This doubly magic configuration is the most likely to have a very long half-life. The next lighter doubly magic spherical nucleus is lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
-208, the heaviest known stable nucleus and most stable heavy metal.
Discussion
The valley of stability can be helpful in interpreting and understanding properties of nuclear decay processes such as decay chains andnuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
.
 Radioactive decay often proceeds via a sequence of steps known as a decay chain. For example, 238U decays to 234Th which decays to 234mPa and so on, eventually reaching 206Pb:
:
With each step of this sequence of reactions, energy is released and the
Radioactive decay often proceeds via a sequence of steps known as a decay chain. For example, 238U decays to 234Th which decays to 234mPa and so on, eventually reaching 206Pb:
:
With each step of this sequence of reactions, energy is released and the decay product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( d ...
s move further down the valley of stability towards the line of beta stability. 206Pb is stable and lies on the line of beta stability.
nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction. They are used for commercial electricity, marine propulsion, weapons production and research. Fissile nuclei (primarily uranium-235 or plutonium-2 ...
are accompanied by the release of neutrons that sustain the chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
. Fission occurs when a heavy nuclide such as uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
absorbs a neutron and breaks into nuclides of lighter elements such as barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
or krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
, usually with the release of additional neutrons. Like all nuclides with a high atomic number, these uranium nuclei require many neutrons to bolster their stability, so they have a large neutron-proton ratio (''N''/''Z''). The nuclei resulting from a fission ( fission products) inherit a similar ''N''/''Z'', but have atomic numbers that are approximately half that of uranium. Isotopes with the atomic number of the fission products and an ''N''/''Z'' near that of uranium or other fissionable nuclei have too many neutrons to be stable; this neutron excess is why multiple free neutrons but no free protons are usually emitted in the fission process, and it is also why many fission product nuclei undergo a long chain of β− decays, each of which converts a nucleus ''N''/''Z'' to (''N'' − 1)/(''Z'' + 1), where ''N'' and ''Z'' are, respectively, the numbers of neutrons and protons contained in the nucleus.
When fission reactions are sustained at a given rate, such as in a liquid-cooled or solid fuel nuclear reactor, the nuclear fuel in the system produces many antineutrinos for each fission that has occurred. These antineutrinos come from the decay of fission products that, as their nuclei progress down a β− decay chain toward the valley of stability, emit an antineutrino along with each β− particle. In 1956, Reines and Cowan exploited the (anticipated) intense flux of antineutrinos from a nuclear reactor in the design of an experiment to detect and confirm the existence of these elusive particles.
See also
*Alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
* Gamma decay
Gamma (; uppercase , lowercase ; ) is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter normally repr ...
* Neutron emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisin ...
* Proton emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a atomic nucleus, nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay ...
* Cluster decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, ...
* Stable nuclide
Stable nuclides are isotopes of a chemical element whose nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The nuclei of such isotopes are not radioactive and unlike radionu ...
* Nuclear shell model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model utilizes the Pauli exclusion principle to model the structure of atomic nuclei in terms of energy levels. The first shell model was proposed by Dmitri Ivanenk ...
* Nuclear drip line
The nuclear drip line is the boundary beyond which atomic nuclei are unbound with respect to the emission of a proton or neutron.
An arbitrary combination of protons and neutrons does not necessarily yield a stable atomic nucleus, nucleus. One ...
References
External links
*The Live Chart of Nuclides - IAEA
with filter on decay type
The Valley of Stability (video)
– a virtual "flight" through 3D representation of the nuclide chart, by CEA (France)
The nuclear landscape: The variety and abundance of nuclei
– Chapter 6 of the book ''Nucleus: A trip into the heart of matter'' by Mackintosh, Ai-Khalili, Jonson, and Pena describes the valley of stability and its implications (Baltimore, Maryland:The Johns Hopkins University Press), 2001. {{ISBN, 0-801 8-6860-2 Nuclear physics Radioactivity Isotopes