Trimix (breathing gas) on:
[Wikipedia]
[Google]
[Amazon]
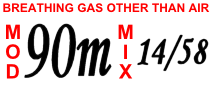

 Trimix is a
Trimix is a


 Technical diver training and certification agencies may differentiate between levels of trimix diving qualifications, The usual distinction is between normoxic trimix and hypoxic trimix, sometimes also called full trimix. The basic distinction is that for hypoxic trimix diving the dive cannot be started on the bottom mix, and procedures for use of a ''travel mix'' for the first part of the descent, and gas switching during the descent to avoid oxygen toxicity are added to the required skills. Longer decompression using a larger variety of mixtures may also complicate procedures. In closed circuit rebreather diving, use of a hypoxic diluent prevents the diver from conducting a diluent flush at shallow depths while breathing from the loop, so that it remains possible at the maximum depth of the dive, where it may be more critical.
Technical diver training and certification agencies may differentiate between levels of trimix diving qualifications, The usual distinction is between normoxic trimix and hypoxic trimix, sometimes also called full trimix. The basic distinction is that for hypoxic trimix diving the dive cannot be started on the bottom mix, and procedures for use of a ''travel mix'' for the first part of the descent, and gas switching during the descent to avoid oxygen toxicity are added to the required skills. Longer decompression using a larger variety of mixtures may also complicate procedures. In closed circuit rebreather diving, use of a hypoxic diluent prevents the diver from conducting a diluent flush at shallow depths while breathing from the loop, so that it remains possible at the maximum depth of the dive, where it may be more critical.
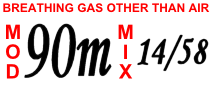
breathing gas
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed ...
consisting of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and is used in deep commercial diving
Commercial diving may be considered an application of professional diving where the diver engages in underwater work for industrial, construction, engineering, maintenance or other commercial purposes which are similar to work done out of the wate ...
, during the deep phase of dives carried out using technical diving
Technical diving (also referred to as tec diving or tech diving) is scuba diving that exceeds the agency-specified limits of recreational diving for non-professional purposes. Technical diving may expose the diver to hazards beyond those normally ...
techniques, and in advanced recreational diving
Recreational diving or sport diving is diving for the purpose of leisure and enjoyment, usually when using scuba equipment. The term "recreational diving" may also be used in contradistinction to "technical diving", a more demanding aspect of ...
.
The helium is included as a substitute for some of the nitrogen, to reduce the narcotic effect of the breathing gas at depth. With a mixture of three gases it is possible to create mixes suitable for different depths or purposes by adjusting the proportions of each gas. Oxygen content can be optimised for the depth to limit the risk of toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
, and the inert component balanced between nitrogen (which is cheap but narcotic) and helium (which is not narcotic and reduces work of breathing, but is more expensive and increases heat loss
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, ...
).
The mixture of helium and oxygen with a 0% nitrogen content is generally known as heliox
Heliox is a breathing gas mixture of helium (He) and oxygen (O2). It is used as a medical treatment for patients with difficulty breathing because mixture generates less resistance than atmospheric air when passing through the airways of the lung ...
. This is frequently used as a breathing gas in deep commercial diving operations, where it is often recycled to save the expensive helium component. Analysis of two-component gases is much simpler than three-component gases.
Function of the helium
The main reason for adding helium to the breathing mix is to reduce the proportions of nitrogen and oxygen below those of air, to allow the gas mix to be breathed safely on deep dives. A lower proportion of nitrogen is required to reducenitrogen narcosis
Narcosis while diving (also known as nitrogen narcosis, inert gas narcosis, raptures of the deep, Martini effect) is a reversible alteration in consciousness that occurs while diving at depth. It is caused by the anesthetic effect of certain g ...
and other physiological effects of the gas at depth. Helium has very little narcotic effect. A lower proportion of oxygen reduces the risk of oxygen toxicity on deep dives.
The lower density of helium reduces breathing resistance at depth. Work of breathing can limit the use of breathing gas mixtures in underwater breathing apparatus, as with increasing depth a point may be reached where work of breathing exceeds the available effort from the diver. Beyond this point accumulation of carbon dioxide will eventually result in severe and debilitating hypercapnia
Hypercapnia (from the Greek ''hyper'' = "above" or "too much" and ''kapnos'' = "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous pro ...
, which, if not corrected quickly, will cause the diver to attempt to breathe faster, exacerbating the work of breathing, which will lead to loss of consciousness and a high risk of drowning.
Because of its low molecular weight, helium enters and leaves tissues more rapidly than nitrogen as the pressure is increased or reduced (this is called on-gassing and off-gassing). Because of its lower solubility, helium does not load tissues as heavily as nitrogen, but at the same time the tissues can not support as high an amount of helium when super-saturated. In effect, helium is a faster gas to saturate and desaturate, which is a distinct advantage in saturation diving
Saturation diving is diving for periods long enough to bring all tissues into equilibrium with the partial pressures of the inert components of the breathing gas used. It is a diving mode that reduces the number of decompressions divers working ...
, but less so in bounce diving, where the increased rate of off-gassing is largely counterbalanced by the equivalently increased rate of on-gassing.
Some divers suffer from hyperbaric arthralgia
Arthralgia (from Greek ''arthro-'', joint + ''-algos'', pain) literally means ''joint pain''. Specifically, arthralgia is a symptom of injury, infection, illness (in particular arthritis), or an allergic reaction to medication.
According to MeSH, ...
(compression arthralgia
Compression arthralgia is pain in the joints caused by exposure to high ambient pressure at a relatively high rate of compression, experienced by underwater divers. Also referred to in the U.S. Navy Diving Manual as compression pains.
Compressio ...
) during descent, and trimix has been shown to help the symptoms of compression.
Disadvantages of the helium
Helium conducts heat six times faster than air, so helium-breathing divers often carry a separate supply of a different gas to inflate drysuits. This is to avoid the risk of hypothermia caused by using helium as inflator gas.Argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
, carried in a small, separate tank connected only to the inflator of the drysuit, is preferred to air, since air conducts heat 50% faster than argon. Dry suits (if used together with a buoyancy compensator) still require a minimum of inflation to avoid "squeezing", i.e. damage to skin caused by pressurizing dry suit folds.
Helium dissolves into tissues (this is called on-gassing) more rapidly than nitrogen as the ambient pressure is increased. A consequence of the higher loading in some tissues is that many decompression algorithms require deeper decompression stops than a similar decompression dive using air, and helium is more likely to come out of solution and cause decompression sickness
Decompression sickness (abbreviated DCS; also called divers' disease, the bends, aerobullosis, and caisson disease) is a medical condition caused by dissolved gases emerging from solution as bubbles inside the body tissues during decompressio ...
following a fast ascent.
In addition to physiological disadvantages, the use of trimix also has economic and logistic disadvantages. The price of helium increased by over 51% between the years 2000 and 2011. This price increase affects open-circuit divers more than closed-circuit divers due to the larger volume of helium consumed on a typical trimix dive. Additionally, as trimix fills require a more elaborate blending and compressor setup than less complex air and nitrox fills, there are fewer trimix filling stations. The relative scarcity of trimix filling stations may necessitate going far out of one's way in order to procure the necessary mix for a deep dive that requires the gas.
Advantages of controlling the oxygen fraction
Lowering the oxygen content increases themaximum operating depth
In underwater diving activities such as saturation diving, technical diving and nitrox diving, the maximum operating depth (MOD) of a breathing gas is the depth below which the partial pressure of oxygen (pO2) of the gas mix exceeds an acceptable ...
and duration of the dive before which oxygen toxicity becomes a limiting factor. Most trimix divers limit their working oxygen partial pressure O2to 1.4 bar and may reduce the PO2 further to 1.3 bar or 1.2 bar depending on the depth, the duration and the kind of breathing system used. A maximum oxygen partial pressure of 1.4 bar for the active sectors of the dive, and 1.6 bar for decompression stops is recommended by several recreational and technical diving certification agencies for open circuit, and 1.2 bar or 1.3 bar as maximum for the active sectors of a dive on closed-circuit rebreather. Increasing the oxygen fraction in a trimix to be used as a decompression gas can accelerate decompression with a lowered risk of isobaric counter diffusion complications.
Advantages of keeping some nitrogen in the mix
Retaining nitrogen in trimix can contribute to the prevention of High Pressure Nervous Syndrome, a problem that can occur when breathingheliox
Heliox is a breathing gas mixture of helium (He) and oxygen (O2). It is used as a medical treatment for patients with difficulty breathing because mixture generates less resistance than atmospheric air when passing through the airways of the lung ...
at depths beyond about . Nitrogen is also much less expensive than helium.
Naming conventions
The term trimix implies that the gas has three functional components, which are helium, nitrogen and oxygen. Since the nitrogen and all or part of the oxygen is usually provided from air, the other components of ordinary atmospheric air are generally ignored. Conventionally, the composition of a mix is specified by its oxygen percentage, helium percentage and optionally the balance percentage, nitrogen, in that order. For example, a mix named "trimix 10/70" or trimix 10/70/20, consisting of 10% oxygen, 70% helium, 20% nitrogen is suitable for a dive. Hyperoxic trimix is sometimes referred to as Helitrox, TriOx, or HOTx (High Oxygen Trimix) with the "x" in HOTx representing the mixture's fraction of helium as a percentage. The basic term Trimix is sufficient, modified as appropriate with the terms hypoxic, normoxic and hyperoxic, and the usual forms for indicating constituent gas fraction, to describe any possible ratio of gases, but theNational Association of Underwater Instructors
The National Association of Underwater Instructors (NAUI Worldwide) is a non-profit association of scuba instructors. It primarily serves as a recreational dive certification and membership organization established to provide international div ...
(NAUI) uses the term "helitrox" for hyperoxic 26/17 Trimix, i.e. 26% oxygen, 17% helium, 57% nitrogen. Helitrox requires decompression stops similar to Nitrox-I (EAN32) and has a maximum operating depth
In underwater diving activities such as saturation diving, technical diving and nitrox diving, the maximum operating depth (MOD) of a breathing gas is the depth below which the partial pressure of oxygen (pO2) of the gas mix exceeds an acceptable ...
of , where it has an equivalent narcotic depth of . This allows diving throughout the usual recreational range, while decreasing decompression obligation and narcotic effects compared to air. GUE and UTD also promote hyperoxic trimix for this depth range, but prefer the term "TriOx".
Applications
In open-circuit scuba, two classes of trimix are commonly used: '' normoxic'' trimix—with a minimum PO2 at the surface of 0.18 and ''hypoxic
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the t ...
'' trimix—with a PO2 less than 0.18 at the surface. A normoxic mix such as "19/30" is used in the depth range; a hypoxic mix such as "10/50" is used for deeper diving, as a bottom gas only, and cannot safely be breathed at shallow depths where the PO2 is less than 0.18 bar.
In fully closed-circuit rebreathers that use trimix diluents, the mix in the breathing loop can be '' hyperoxic'' (meaning more oxygen than in air, as in enriched air nitrox) in shallow water, because the rebreather automatically adds oxygen to maintain a specific partial pressure of oxygen. Hyperoxic trimix is also sometimes used on open circuit scuba, to reduce decompression obligations.
Blending
Gas blending
Gas blending is the process of mixing gases for a specific purpose where the composition of the resulting mixture is specified and controlled.
A wide range of applications include scientific and industrial processes, food production and storage and ...
of trimix generally involves mixing helium and oxygen with air to the desired proportions and pressure. Two methods are in common use:
Partial pressure blending is done by decanting oxygen and helium into the diving cylinder
A diving cylinder or diving gas cylinder is a gas cylinder used to store and transport high pressure gas used in diving operations. This may be breathing gas used with a scuba set, in which case the cylinder may also be referred to as a sc ...
and then topping up the mix with air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
from a diving air compressor
A diving air compressor is a gas compressor that can provide breathing air directly to a surface-supplied diver, or fill diving cylinders with high-pressure air pure enough to be used as a breathing gas. A low pressure diving air compressor us ...
. To ensure an accurate mix, after each helium and oxygen transfer, the mix is allowed to cool, its pressure is measured and further gas is decanted until the correct pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
is achieved. This process often takes hours and is sometimes spread over days at busy blending stations. Corrections can be made for temperature effect, but this requires accurate monitoring of the temperature of the mixture inside the cylinder, which is generally not available.
A second method called 'continuous blending' is done by mixing oxygen and helium into the intake air of a compressor. The oxygen and helium are fed into mixing tubes in the intake air stream using flow meters or analysis of the oxygen content after oxygen addition and before and after the helium addition, and the oxygen and helium flows adjusted accordingly. On the high pressure side of the compressor a regulator or bleed orifice is used to reduce pressure of a sample flow and the trimix is analyzed (preferably for both helium and oxygen) so that the fine adjustment to the intake gas flows can be made. The benefit of such a system is that the helium delivery tank pressure need not be as high as that used in the partial pressure method of blending and residual gas can be 'topped up' to best mix after the dive. This is important mainly because of the high cost of helium. Drawbacks may be that the high heat of compression of helium results in the compressor overheating, especially in hot weather. Temperature of the trimix entering the analyser should be kept constant for best reliability of the analysis, and the analyser should be calibrated at ambient temperature before use. The mixing tube is a very simple device, and DIY versions of the continuous blend units can be made for a relatively low cost compared to the cost of analysers and compressor.
Choice of mixture composition
The ratio of gases in a particular mix is chosen to give a safemaximum operating depth
In underwater diving activities such as saturation diving, technical diving and nitrox diving, the maximum operating depth (MOD) of a breathing gas is the depth below which the partial pressure of oxygen (pO2) of the gas mix exceeds an acceptable ...
and comfortable equivalent narcotic depth for the planned dive. Safe limits for mix of gases in trimix are generally accepted to be a maximum partial pressure of oxygen (PO2—see Dalton's law
Dalton's law (also called Dalton's law of partial pressures) states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. This empirical law was observed by Joh ...
) of 1.0 to 1.6 bar and maximum equivalent narcotic depth of . At , "12/52" has a PO2 of 1.3 bar and an equivalent narcotic depth of .
"Standard" mixes
Although theoretically trimix can be blended with almost any combination of helium and oxygen, a number of "standard" mixes have evolved (such as 21/35, 18/45 and 15/55—see ''Naming conventions''). Most of these mixes originated from starting by decanting a given pressure of helium into an empty cylinder, and then topping up the mix with 32% nitrox. The "standard" mixes evolved because of three coinciding factors — the desire to keep the equivalent narcotic depth (END) of the mix at approximately , the requirement to keep the partial pressure of oxygen at 1.4 ATA or below at the deepest point of the dive, and the fact that many dive shops stored standard 32% nitrox in banks, which simplifies mixing. The use of standard mixes makes it relatively easy to top up diving cylinders after a dive using residual mix — only helium and banked nitrox are needed to top up the residual gas from the last fill. The method of mixing a known nitrox mix with helium allows analysis of the fractions of each gas using only an oxygen analyser, since the ratio of the oxygen fraction in the final mix to the oxygen fraction in the initial nitrox gives the fraction of nitrox in the final mix, hence the fractions of the three components are easily calculated. It is demonstrably true that the END of a nitrox-helium mixture at itsmaximum operating depth
In underwater diving activities such as saturation diving, technical diving and nitrox diving, the maximum operating depth (MOD) of a breathing gas is the depth below which the partial pressure of oxygen (pO2) of the gas mix exceeds an acceptable ...
(MOD) is equal to the MOD of the nitrox alone.
Heliair
Heliair is abreathing gas
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed ...
consisting of mixture of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
and is often used during the deep phase of dives carried out using technical diving
Technical diving (also referred to as tec diving or tech diving) is scuba diving that exceeds the agency-specified limits of recreational diving for non-professional purposes. Technical diving may expose the diver to hazards beyond those normally ...
techniques. This term, first used by Sheck Exley
Sheck Exley (April 1, 1949 – April 6, 1994) was an American cave diver. He is widely regarded as one of the pioneers of cave diving, and he wrote two major books on the subject: '' Basic Cave Diving: A Blueprint for Survival'' and ''Caverns Mea ...
, is mostly used by Technical Diving International
Technical Diving International (TDI) claims to be the largest technical diving certification agency in the world, and one of the first agencies to offer mixed gas and rebreather training. TDI specializes in more advanced Scuba diving techniq ...
(TDI).
It is easily blended from helium and air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
and so has a fixed 21:79 ratio of oxygen to nitrogen with the balance consisting of a variable amount of helium. It is sometimes referred to as "poor man's trimix", because it is much easier to blend than trimix blends with variable oxygen content, since all that is required is to insert the requisite partial pressure of helium, and then top up with air from a conventional compressor. The more complicated (and dangerous) step of adding pure oxygen at pressure required to blend trimix is absent when blending heliair.
Heliair blends are similar to the standard Trimix blends made with helium and Nitrox 32, but with a deeper END at MOD.
Heliair will always have less than 21% oxygen, and will be hypoxic (less than 17% oxygen) for mixes with more than 20% helium.
History as a diving gas
*1919: ProfessorElihu Thomson
Elihu Thomson (March 29, 1853 – March 13, 1937) was an English-born American engineer and inventor who was instrumental in the founding of major electrical companies in the United States, the United Kingdom and France.
Early life
He was bor ...
speculates that helium could be used instead of nitrogen to reduce the breathing resistance at great depth. Heliox
Heliox is a breathing gas mixture of helium (He) and oxygen (O2). It is used as a medical treatment for patients with difficulty breathing because mixture generates less resistance than atmospheric air when passing through the airways of the lung ...
was used with air tables resulting in a high incidence of decompression sickness, so the use of helium was discontinued.
*1924: The US Navy
The United States Navy (USN) is the maritime service branch of the United States Armed Forces and one of the eight uniformed services of the United States. It is the largest and most powerful navy in the world, with the estimated tonnage ...
begins examining helium's potential usage and by the mid-1920s lab animals were exposed to experimental chamber dives using heliox. Soon, human subjects breathing heliox 20/80 (20% oxygen, 80% helium) had been successfully decompressed from deep dives.
*1937: Several test dives are conducted with helium mixtures, including salvage diver Max "Gene" Nohl's dive to 127 meters.
*1939: US Navy uses heliox in USS ''Squalus'' salvage operation. Heliox usage, coupled with the absence of decrement in co-ordination and cognitive function in the salvage divers, confirms Behnke's theory of nitrogen narcosis.
*1965: Nic Flemming's work to study sand ribbons in the English Channel becomes the first to compare diver performance while breathing air and heliox in the open water.
*1963: First saturation dives using trimix as part of Project Genesis.
*1970: Hal Watts recovers two bodies at Mystery Sink (126 m).
*1979: A research team headed by Peter B. Bennett at the Duke University Medical Center Hyperbaric Laboratory begins the "Atlantis Dive Series" which proves the mechanisms behind the use of trimix to prevent High Pressure Nervous Syndrome symptoms.
*1983: Cave diver Jochen Hasenmayer
Jochen Hasenmayer (born 28 October 1941 in Pforzheim, Germany) is a German speleologist and cave diver from Birkenfeld in Baden-Württemberg, whose spectacular dives have frequently made headlines.
Cave diving
Hasenmayer began his cave divin ...
uses heliox to a depth of 212 meters. Depth is later repeated by Sheck Exley
Sheck Exley (April 1, 1949 – April 6, 1994) was an American cave diver. He is widely regarded as one of the pioneers of cave diving, and he wrote two major books on the subject: '' Basic Cave Diving: A Blueprint for Survival'' and ''Caverns Mea ...
in 1987.
*1987: First mass use of trimix and heliox: Wakulla Springs
Wakulla Springs is located south of Tallahassee, Florida and east of Crawfordville in Wakulla County, Florida at the crossroads of State Road 61 and State Road 267. It is protected in the Edward Ball Wakulla Springs State Park.
Description ...
Project. Exley teaches non-commercial divers in relation to trimix usage in cave diving.
*1991: Billy Deans commences teaching of trimix diving for recreational diving. Tom Mount develops first trimix training standards (IANTD #REDIRECT International Association of Nitrox and Technical Divers
The International Association of Nitrox and Technical Divers (IANTD) is a scuba diving organization concerned with certification and training in recreational diving, technical ...
). Use of trimix spreads rapidly to North East American wreck diving community.
*1992: The National Oceanographic and Atmospheric Administration (NOAA) develops "Monitor Mix" for dives to the USS '' Monitor''. This mix became NOAA Trimix I, with decompression tables designed by Bill Hamilton published in the NOAA Diving Manual.
*1992: NOAA obtains training from Key West Divers to conduct the first NOAA-sponsored trimix dives on the wreck of the USS ''Monitor'' off Cape Hatteras, NC.
*1994: Combined UK/USA team, including wreck divers John Chatterton
John Chatterton (born 1951) is an American wreck diver. Together with Richie Kohler, he was one of the co-hosts for the History Channel’s ''Deep Sea Detectives'', for 57 episodes of the series. He is also a consultant to the film and televis ...
and Gary Gentile
Gary Gentile (born 1946) is an American author and pioneering technical diver.
Diving
Gary Gentile is a wreck diver. It has been suggested that Gary Gentile may be the most experienced wreck diver in the world. He has dived on the wreck of the ...
, successfully completes a series of wreck dives on the '' RMS Lusitania'' expedition to a depth of 100 meters using trimix.
*1994: Sheck Exley
Sheck Exley (April 1, 1949 – April 6, 1994) was an American cave diver. He is widely regarded as one of the pioneers of cave diving, and he wrote two major books on the subject: '' Basic Cave Diving: A Blueprint for Survival'' and ''Caverns Mea ...
and Jim Bowden use "heliair" at Zacaton in the first attempt to make an open circuit scuba dive to 1000 ft. Exley, at the time holding the world record for an 881-foot dive, passes out and dies around 900 feet; Bowden aborts at 925 feet and survives despite several life-threatening obstacles.
*2001: The Guinness Book of records recognises John Bennett as the first scuba diver to dive to , using trimix.
*2005: David Shaw sets depth record for using a trimix rebreather
A rebreather is a breathing apparatus that absorbs the carbon dioxide of a user's breathing, exhaled breath to permit the rebreathing (recycling) of the substantially unused oxygen content, and unused inert content when present, of each breath. ...
, and dies while repeating the dive to attempt to recover the body of another diver.
*2015: The United States Navy Experimental Diving Unit
The United States Navy Experimental Diving Unit (NEDU or NAVXDIVINGU) is the primary source of diving and hyperbaric operational guidance for the US Navy. It is located within the Naval Support Activity Panama City in Panama City Beach, Bay Count ...
shows that bounce dives using trimix are not more decompression efficient than dives on heliox.
Training and certification
 Technical diver training and certification agencies may differentiate between levels of trimix diving qualifications, The usual distinction is between normoxic trimix and hypoxic trimix, sometimes also called full trimix. The basic distinction is that for hypoxic trimix diving the dive cannot be started on the bottom mix, and procedures for use of a ''travel mix'' for the first part of the descent, and gas switching during the descent to avoid oxygen toxicity are added to the required skills. Longer decompression using a larger variety of mixtures may also complicate procedures. In closed circuit rebreather diving, use of a hypoxic diluent prevents the diver from conducting a diluent flush at shallow depths while breathing from the loop, so that it remains possible at the maximum depth of the dive, where it may be more critical.
Technical diver training and certification agencies may differentiate between levels of trimix diving qualifications, The usual distinction is between normoxic trimix and hypoxic trimix, sometimes also called full trimix. The basic distinction is that for hypoxic trimix diving the dive cannot be started on the bottom mix, and procedures for use of a ''travel mix'' for the first part of the descent, and gas switching during the descent to avoid oxygen toxicity are added to the required skills. Longer decompression using a larger variety of mixtures may also complicate procedures. In closed circuit rebreather diving, use of a hypoxic diluent prevents the diver from conducting a diluent flush at shallow depths while breathing from the loop, so that it remains possible at the maximum depth of the dive, where it may be more critical.
See also
* * * * *References
{{DEFAULTSORT:Trimix (Breathing Gas) Breathing gases Helium