Tissue transglutaminase on:
[Wikipedia]
[Google]
[Amazon]
Tissue transglutaminase (abbreviated as tTG or TG2) is a 78-kDa, calcium-dependent
 Recent studies have suggested that interferon-γ may serve as an activator of extracellular tTG in the small intestine; these studies have a direct implication to the pathogenesis of celiac disease. Activation of tTG has been shown to be accompanied by large conformational changes, switching from a compact (inactive) to an extended (active) conformation. (see Figure 3)
Recent studies have suggested that interferon-γ may serve as an activator of extracellular tTG in the small intestine; these studies have a direct implication to the pathogenesis of celiac disease. Activation of tTG has been shown to be accompanied by large conformational changes, switching from a compact (inactive) to an extended (active) conformation. (see Figure 3)
 In the
In the 
Endomysial antibodies
* A collection of substrates and interaction partners of TG2 is accessible in th
TRANSDAB
an interactive transglutaminase substrate database. {{Autoantigens EC 2.3.2 Autoantigens
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
() of the protein-glutamine γ-glutamyltransferases family (or simply transglutaminase
Transglutaminases are enzymes that in nature primarily catalyze the formation of an isopeptide bond between γ-carboxamide groups ( -(C=O)NH2 ) of glutamine residue side chains and the ε-amino groups ( -NH2 ) of lysine residue side ...
family). Like other transglutaminases, it crosslinks protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s between an ε-amino
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
group of a lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated − ...
residue and a γ-carboxamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
group of glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
residue, creating an inter- or intramolecular bond that is highly resistant to proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
(protein degradation). Aside from its crosslinking function, tTG catalyzes other types of reactions including deamidation, GTP-binding/hydrolyzing, and isopeptidase activities. Unlike other members of the transglutaminase family, tTG can be found both in the intracellular and the extracellular spaces of various types of tissues and is found in many different organs including the heart, the liver, and the small intestine. Intracellular tTG is abundant in the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
but smaller amounts can also be found in the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
* Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
and the mitochondria
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used ...
. Intracellular tTG is thought to play an important role in apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes in ...
. In the extracellular space, tTG binds to proteins of the extracellular matrix (ECM), binding particularly tightly to fibronectin
Fibronectin is a high- molecular weight (~500-~600 kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as coll ...
. Extracellular tTG has been linked to cell adhesion, ECM stabilization, wound healing, receptor signaling, cellular proliferation, and cellular motility.
tTG is the autoantigen
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". ...
in celiac disease
Coeliac disease (British English) or celiac disease (American English) is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barle ...
, a lifelong illness in which the consumption of dietary gluten
Gluten is a structural protein naturally found in certain cereal grains. Although "gluten" often only refers to wheat proteins, in medical literature it refers to the combination of prolamin and glutelin proteins naturally occurring in all grai ...
causes a pathological immune response resulting in the inflammation of the small intestine and subsequent villous atrophy. It has also been implicated in the pathophysiology of many other diseases, including such as many different cancers and neurogenerative diseases.
Structure
Gene
The human tTG gene is located on the 20th chromosome (20q11.2-q12).Protein
TG2 is a multifunctional enzyme that belongs totransglutaminases
Transglutaminases are enzymes that in nature primarily catalyze the formation of an isopeptide bond between γ-carboxamide groups ( -(C=O)NH2 ) of glutamine residue side chains and the ε-amino groups ( -NH2 ) of lysine residue side ch ...
which catalyze the crosslinking of proteins by epsilon-(gamma-glutamyl)lysine isopeptide bonds. Similarly to other transglutaminases, tTG consists of a GTP/ GDP binding site, a catalytic domain
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) ...
, two beta barrel
In protein structures, a beta barrel is a beta sheet composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand (hydrogen bond). Beta-strands in many beta-barrels are ...
and a beta-sandwich
Beta-sandwich, β-sandwich domains consisting of 80 to 350 amino acids occur commonly in proteins. They are characterized by two opposing antiparallel beta sheets (β-sheets). The number of strands found in such domains may differ from one protein ...
. Crystal structures of TG2 with bound GDP, GTP, or ATP have demonstrated that these forms of TG2 adopt a "closed" conformation, whereas TG2 with the active site occupied by an inhibitory gluten peptide mimic or other similar inhibitors adopts an "open" conformation. In the open conformation the four domains of TG2 are arranged in an extended configuration, allowing for catalytic activity, whereas in the closed conformation the two C-terminal domains are folded in on the catalytic core domain which includes the residue Cys-277. The N-terminal domain
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
only shows minor structural changes between the two different conformations.
Mechanism
The catalytic mechanism for crosslinking in human tTG involves thethiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
group from a Cys residue in the active site of tTG. The thiol group attacks the carboxamide of a glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
residue on the surface of a protein or peptide substrate, releasing ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
, and producing a thioester intermediate. The thioester intermediate can then be attacked by the surface amine of a second substrate (typically from a lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated − ...
residue). The end product of the reaction is a stable isopeptide bond between the two substrates (i.e. crosslinking). Alternatively, the thioester intermediate can be hydrolyzed, resulting in the net conversion of the glutamine residue to glutamic acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synt ...
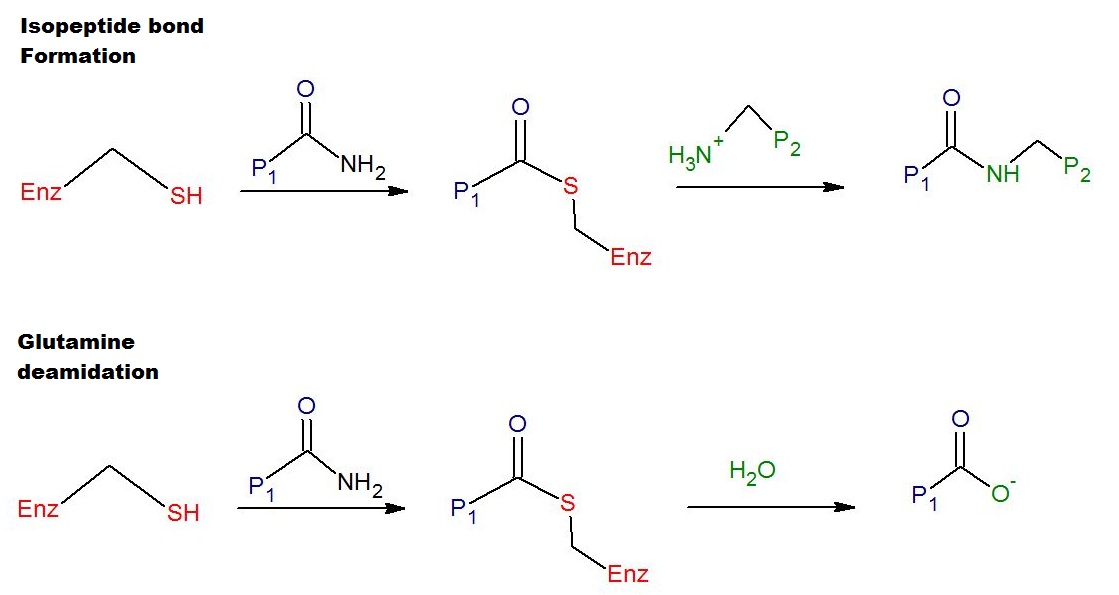
(i.e. deamidation). The deamidation of glutamine residues catalyzed by tTG is thought to be linked to the pathological immune response to gluten in celiac disease. A schematic for the crosslinking and the deamidation reactions is provided in Figure 1.

Regulation
The expression of tTG is regulated at the transcriptional level depending on complex signal cascades. Once synthesized, most of the protein is found in the cytoplasm, plasma membrane and ECM, but a small fraction is translocated to thenucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
* Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
, where it participates in the control of its own expression through the regulation of transcription factor
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The f ...
s.
Crosslinking activity by tTG requires the binding of Ca2+ ions. Multiple Ca2+ can bind to a single tTG molecule. Specifically, tTG binds up to 6 calcium ions at 5 different binding sites. Mutations to these binding sites causing lower calcium affinity, decrease the enzyme's transglutaminase activity. In contrast, the binding of one molecule of GTP or GDP inhibits the crosslinking activity of the enzyme. Therefore, intracellular tTG is mostly inactive due to the relatively high concentration of GTP/GDP and the low levels of calcium inside the cell. Although extracellular tTG is expected to be active due to the low concentration of guanine
Guanine () ( symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is ...
nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules with ...
and the high levels of calcium in the extracellular space, evidence has shown that extracellular tTG is mostly inactive. Recent studies suggest that extracellular tTG is kept inactive by the formation of a disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
bond between two vicinal cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
residues, namely Cys 370 and Cys 371. When this disulfide bond forms, the enzyme remains in an open confirmation but becomes catalytically inactive. The, oxidation/reduction of the disulfide bond serves as a third allosteric regulatory mechanism (along with GTP/GDP and Ca2+) for the activation of tTG. Thioredoxin-1 has been shown to activate extracellular tTG by reducing the disulfide bond. Another disuplhide bond can form in tTG, between the residues Cys-230 and Cys-370. While this bond does not exist in the enzyme's native state, it appears when the enzyme is inactivated via oxidation. The presence of calcium protects against the formation of both disulfide bonds, thus making the enzyme more resistant to oxidation.
 Recent studies have suggested that interferon-γ may serve as an activator of extracellular tTG in the small intestine; these studies have a direct implication to the pathogenesis of celiac disease. Activation of tTG has been shown to be accompanied by large conformational changes, switching from a compact (inactive) to an extended (active) conformation. (see Figure 3)
Recent studies have suggested that interferon-γ may serve as an activator of extracellular tTG in the small intestine; these studies have a direct implication to the pathogenesis of celiac disease. Activation of tTG has been shown to be accompanied by large conformational changes, switching from a compact (inactive) to an extended (active) conformation. (see Figure 3)
 In the
In the extracellular matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide s ...
, TG2 is "turned off", due primarily to the oxidizing activity of endoplasmic reticulum protein 57 (ERp57). Thus, tTG is allosterically regulated by two separate proteins, Erp57 and TRX-1. (See Figure 4).

Function
tTG is expressed ubiquitously and is present in various cellular compartments, such as the cytosol, the nucleus, and the plasma membrane. It requirescalcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
as a cofactor for transamidation activity. Transcription is increased by retinoic acid. Among its many supposed functions, it appears to play a role in wound healing
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
In undamaged skin, the epidermis (surface, epithelial layer) and dermis (deeper, connective layer) form a protective barrier again ...
, apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes in ...
, and extracellular matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide s ...
development as well as differentiation and cell adhesion
Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indire ...
. It has been noted that tTG may have very different activity in different cell types. For example, in neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa ...
s, tTG supports the survival of cells subjected to injury whereas in astrocyte
Astrocytes (from Ancient Greek , , "star" + , , "cavity", "cell"), also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of e ...
s knocking out the gene expression for tTG is beneficial to cell survival.
tTG is thought to be involved in the regulation of the cytoskeleton by crosslinking various cytoskeletal proteins including myosin, actin, and spectrin. Evidence shows that intracellular tTG crosslinks itself to myosin. It is also believed that tTG may stabilize the structure of the dying cells during apoptosis by polymerizing the components of the cytoskeleton, therefore preventing the leakage of the cellular contents into the extracellular space.
tTG also has GTPase activity: In the presence of GTP, it suggested to function as a G protein participating in signaling processes. Besides its transglutaminase activity, tTG is proposed to also act as kinase, and protein disulfide isomerase, and deamidase. This latter activity is important in the deamidation of gliadin peptides, thus playing important role in the pathology of coeliac disease
Coeliac disease (British English) or celiac disease (American English) is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barle ...
.
tTG also presents PDI (Protein Disulfide Isomerase) activity. Based on its PDI activity, tTG plays an important role in the regulation of proteostasis, by catalyzing the trimerization of HSF1 (Heat Shock Factor 1) and thus the body's response to heat shock. In the absence of tTG, the response to heat shock is impaired since the necessary trimer is not formed.
Clinical significance
tTG is the most comprehensively studied transglutaminase and has been associated with many diseases. However, none of these diseases are related to an enzyme deficiency. Indeed, thus far no disease has been attributed to the lack of tTG activity and this has been attested through the study of tTG knockout mice.Celiac Disease
tTG is best known for its link withceliac disease
Coeliac disease (British English) or celiac disease (American English) is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barle ...
. It was first associated with celiac disease in 1997 when the enzyme was found to be the antigen recognized by the antibodies specific to celiac. Anti-transglutaminase antibodies result in a form of gluten sensitivity
Non-celiac gluten sensitivity (NCGS) or gluten sensitivity is "a clinical entity induced by the ingestion of gluten leading to intestinal and/or extraintestinal symptoms that improve once the gluten-containing foodstuff is removed from the diet, a ...
in which a cellular response to ''Triticeae'' glutens that are crosslinked to tTG are able to stimulate transglutaminase specific B-cell
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted o ...
responses that eventually result in the production of anti-transglutaminase antibodies IgA and IgG. tTG specifically deamidates the glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
residues creating epitopes that increase the binding affinity of the gluten
Gluten is a structural protein naturally found in certain cereal grains. Although "gluten" often only refers to wheat proteins, in medical literature it refers to the combination of prolamin and glutelin proteins naturally occurring in all grai ...
peptide to the antigen presenting T cells
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell re ...
, initiating an adaptive immune response.
Cancer
Recent studies suggest that tTG also plays a role ininflammation
Inflammation (from la, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molec ...
and tumor biology. tTG expression is elevated in multiple cancer cell types and is implicated in drug resistance and metastasis due to its ability to promote mesenchymal transition and stem cell like properties. In its GTP bound form, tTG contributes to cancer cell survival and appears to be a cancer driver. tTG is upregulated in cancer cells and tissues in many cancer types, including leukemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ...
, breast cancer
Breast cancer is cancer that develops from breast tissue. Signs of breast cancer may include a lump in the breast, a change in breast shape, dimpling of the skin, milk rejection, fluid coming from the nipple, a newly inverted nipple, or ...
, prostate cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that su ...
, pancreatic cancer
Pancreatic cancer arises when cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a mass. These cancerous cells have the ability to invade other parts of the body. A number of types of pancr ...
and cervical cancer
Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal ...
. Higher tTG expression also correlates with higher instances of metastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, the ...
, chemotherapy resistance, lower survival rates and generally poor prognosis. Cancer cells can be killed by increasing calcium levels through the activation of tTG transamidation activity. Preclinical trials have showed promise in using tTG inhibitors as anti-cancer therapeutic agents. However, other studies have noted that tTG transamidation activity could be linked to the inhibition of tumor cell invasiveness.
Other Diseases
tTG is believed to contribute to several neurodegenerative disorders includingAlzheimer
Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As t ...
, Parkinson and Huntington diseases by affecting transcription, differentiation and migration and adhesion . Such neurological diseases are characterized in part by the abnormal aggregation of proteins due to the increased activity of protein crosslinking in the affected brain. Additionally, specific proteins associated with these disorders have been found to be in vivo and in vitro substrates of tTG.
Although tTG is up regulated in the areas of the brain affected by Huntington's disease, a recent study showed that increasing levels of tTG do not affect the onset and/or progression of the disease in mice.
Recent studies show that tTG may not be involved in AD as studies show it is associated with erythrocyte lysis and is a consequence of the disease rather than a cause.
tTG has also been linked to the pathogenesis of fibrosis
Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of perma ...
in various organs including the lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of ...
and the kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blo ...
. Specifically, in kidney fibrosis, tTG contributes to the stabilization and accumulation of the ECM affecting TGF beta
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other ...
activity.
Diagnostic
Serology
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection (against a given mic ...
for anti-tTG antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of ...
has superseded older serological tests (anti-endomysium, anti-gliadin, and anti-reticulin) and has a strong sensitivity (99%) and specificity (>90%) for identifying celiac disease. Modern anti-tTG assays rely on a human recombinant protein as an antigen.
Therapeutic
It's still experimental to use tTG as a form of surgical glue. It is also being studied as an attenuator ofmetastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, the ...
in certain tumors. tTG shows promise as a potential therapeutic target to treat cardiac fibrosis, through the activity of a highly selective tTG inhibitor
Inhibitor or inhibition may refer to:
In biology
* Enzyme inhibitor, a substance that binds to an enzyme and decreases the enzyme's activity
* Reuptake inhibitor, a substance that increases neurotransmission by blocking the reuptake of a neurotra ...
. tTG inhibitors have also been shown to inhibit the formation of toxic inclusions related to neurodegenerative diseases. This indicates that tTG inhibitors could also serve as a tool to mitigate the progression of tTG brain related diseases.
Interactions
TG2 participates in both enzymatic and non-enzymaticinteractions
Interaction is action that occurs between two or more objects, with broad use in philosophy and the sciences. It may refer to:
Science
* Interaction hypothesis, a theory of second language acquisition
* Interaction (statistics)
* Interactions ...
. Enzymatic interactions are formed between TG2 and its substrate proteins containing the glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
donor and lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated − ...
donor groups in the presence of calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
. Substrates of TG2 are known to affect TG2 activity, which enables it to subsequently execute diverse biological functions in the cell. However, the importance of non-enzymatic interactions in regulating TG2 activities is yet to be revealed. Recent studies indicate that non-enzymatic interactions play physiological roles and enable diverse TG2 functions in a context-specific manner.
References
External links
Endomysial antibodies
* A collection of substrates and interaction partners of TG2 is accessible in th
TRANSDAB
an interactive transglutaminase substrate database. {{Autoantigens EC 2.3.2 Autoantigens