Tempering (metallurgy) on:
[Wikipedia]
[Google]
[Amazon]
 Tempering is a process of heat treating, which is used to increase the
Tempering is a process of heat treating, which is used to increase the
 Tempering is a heat treatment technique applied to ferrous
Tempering is a heat treatment technique applied to ferrous
 Tempering is a process of heat treating, which is used to increase the
Tempering is a process of heat treating, which is used to increase the toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
-based alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
s. Tempering is usually performed after hardening, to reduce some of the excess hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures.
Introduction
 Tempering is a heat treatment technique applied to ferrous
Tempering is a heat treatment technique applied to ferrous alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
s, such as steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
or cast iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impuri ...
, to achieve greater toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
of the alloy. The reduction in hardness is usually accompanied by an increase in ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
, thereby decreasing the brittleness of the metal. Tempering is usually performed after quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as ...
, which is rapid cooling of the metal to put it in its hardest state. Tempering is accomplished by controlled heating of the quenched work-piece to a temperature below its "lower critical temperature". This is also called the lower transformation temperature or lower arrest (A1) temperature; the temperature at which the crystalline phases of the alloy, called ferrite and cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, ...
, begin combining to form a single-phase solid solution referred to as austenite
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 100 ...
. Heating above this temperature is avoided, so as not to destroy the very-hard, quenched microstructure, called martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation.
Properties
M ...
.''Steel metallurgy for the non-metallurgist'' By John D. Verhoeven - ASM International 2007 Page 99-105
Precise control of time and temperature during the tempering process is crucial to achieve the desired balance of physical properties. Low tempering temperatures may only relieve the internal stresses, decreasing brittleness while maintaining a majority of the hardness. Higher tempering temperatures tend to produce a greater reduction in the hardness, sacrificing some yield strength
In materials science and engineering, the yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and wi ...
and tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials ...
for an increase in elasticity and plasticity. However, in some low alloy steels, containing other elements like chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
and molybdenum, tempering at low temperatures may produce an increase in hardness, while at higher temperatures the hardness will decrease. Many steels with high concentrations of these alloying elements behave like precipitation hardening alloys, which produces the opposite effects under the conditions found in quenching and tempering, and are referred to as maraging steels.
In carbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, coba ...
s, tempering alters the size and distribution of carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of t ...
s in the martensite, forming a microstructure called "tempered martensite". Tempering is also performed on normalized steels and cast irons, to increase ductility, machinability, and impact strength. Steel is usually tempered evenly, called "through tempering," producing a nearly uniform hardness, but it is sometimes heated unevenly, referred to as "differential tempering," producing a variation in hardness.
History
Tempering is an ancient heat-treating technique. The oldest known example of tempered martensite is apick axe
A pickaxe, pick-axe, or pick is a generally T-shaped hand tool used for prying. Its head is typically metal, attached perpendicularly to a longer handle, traditionally made of wood, occasionally metal, and increasingly fiberglass.
A stand ...
which was found in Galilee
Galilee (; he, הַגָּלִיל, hagGālīl; ar, الجليل, al-jalīl) is a region located in northern Israel and southern Lebanon. Galilee traditionally refers to the mountainous part, divided into Upper Galilee (, ; , ) and Lower Gali ...
, dating from around 1200 to 1100 BC. The process was used throughout the ancient world, from Asia to Europe and Africa. Many different methods and cooling baths for quenching have been attempted during ancient times, from quenching in urine, blood, or metals like mercury or lead, but the process of tempering has remained relatively unchanged over the ages. Tempering was often confused with quenching and, often, the term was used to describe both techniques. In 1889, Sir William Chandler Roberts-Austen
Sir William Chandler Roberts-Austen (3 March 1843, Kennington – 22 November 1902, London) was an English metallurgist noted for his research on the physical properties of metals and their alloys. The austenite class of iron alloys is named aft ...
wrote, "There is still so much confusion between the words "temper," "tempering," and "hardening," in the writings of even eminent authorities, that it is well to keep these old definitions carefully in mind. I shall employ the word tempering in the same sense as softening."
Terminology
In metallurgy, one may encounter many terms that have very specific meanings within the field, but may seem rather vague when viewed from outside. Terms such as "hardness," "impact resistance," "toughness," and "strength" can carry many different connotations, making it sometimes difficult to discern the specific meaning. Some of the terms encountered, and their specific definitions are: * Strength – Resistance to permanent deformation and tearing. Strength, in metallurgy, is still a rather vague term, so is usually divided intoyield strength
In materials science and engineering, the yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and wi ...
(strength beyond which deformation becomes permanent), tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials ...
(the ultimate tearing strength), shear strength (resistance to transverse, or cutting forces), and compressive strength
In mechanics, compressive strength or compression strength is the capacity of a material or structure to withstand loads tending to reduce size (as opposed to tensile strength which withstands loads tending to elongate). In other words, compres ...
(resistance to elastic shortening under a load).
* Toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.fracture
Fracture is the separation of an object or material into two or more pieces under the action of stress. The fracture of a solid usually occurs due to the development of certain displacement discontinuity surfaces within the solid. If a displ ...
, as measured by the Charpy test. Toughness often increases as strength decreases, because a material that bends is less likely to break.
* Hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
– A surface's resistance to scratching, abrasion, or indentation. In conventional metal alloys, there is a linear relation between indentation hardness and tensile strength, which eases the measurement of the latter.
* Brittleness – Brittleness describes a material's tendency to break before bending or deforming either elastically or plastically. Brittleness increases with decreased toughness, but is greatly affected by internal stresses as well.
* Plasticity – The ability to mold, bend or deform in a manner that does not spontaneously return to its original shape. This is proportional to the ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
or malleability of the substance.
* Elasticity – Also called flexibility, this is the ability to deform, bend, compress, or stretch and return to the original shape once the external stress is removed. Elasticity is inversely related to the Young's modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied ...
of the material.
* Impact resistance
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.Wear resistance – Usually synonymous with hardness, this is resistance to
 If steel has been freshly ground, sanded, or polished, it will form an
If steel has been freshly ground, sanded, or polished, it will form an
 Differential tempering is a method of providing different amounts of temper to different parts of the steel. The method is often used in bladesmithing, for making knives and
Differential tempering is a method of providing different amounts of temper to different parts of the steel. The method is often used in bladesmithing, for making knives and
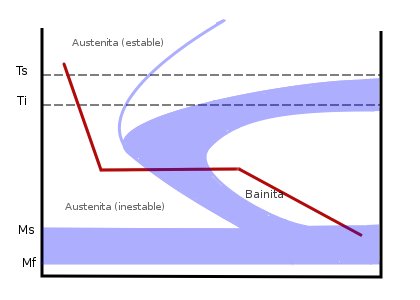 Austempering is a technique used to form pure bainite, a transitional microstructure found between pearlite and martensite. In normalizing, both upper and lower bainite are usually found mixed with pearlite. To avoid the formation of pearlite or martensite, the steel is quenched in a bath of molten metals or salts. This quickly cools the steel past the point where pearlite can form, and into the bainite-forming range. The steel is then held at the bainite-forming temperature, beyond the point where the temperature reaches an equilibrium, until the bainite fully forms. The steel is then removed from the bath and allowed to air-cool, without the formation of either pearlite or martensite.
Depending on the holding-temperature, austempering can produce either upper or lower bainite. Upper bainite is a laminate structure formed at temperatures typically above and is a much tougher microstructure. Lower bainite is a needle-like structure, produced at temperatures below 350 °C, and is stronger but much more brittle. In either case, austempering produces greater strength and toughness for a given hardness, which is determined mostly by composition rather than cooling speed, and reduced internal stresses which could lead to breakage. This produces steel with superior impact resistance. Modern punches and chisels are often austempered. Because austempering does not produce martensite, the steel does not require further tempering.
Austempering is a technique used to form pure bainite, a transitional microstructure found between pearlite and martensite. In normalizing, both upper and lower bainite are usually found mixed with pearlite. To avoid the formation of pearlite or martensite, the steel is quenched in a bath of molten metals or salts. This quickly cools the steel past the point where pearlite can form, and into the bainite-forming range. The steel is then held at the bainite-forming temperature, beyond the point where the temperature reaches an equilibrium, until the bainite fully forms. The steel is then removed from the bath and allowed to air-cool, without the formation of either pearlite or martensite.
Depending on the holding-temperature, austempering can produce either upper or lower bainite. Upper bainite is a laminate structure formed at temperatures typically above and is a much tougher microstructure. Lower bainite is a needle-like structure, produced at temperatures below 350 °C, and is stronger but much more brittle. In either case, austempering produces greater strength and toughness for a given hardness, which is determined mostly by composition rather than cooling speed, and reduced internal stresses which could lead to breakage. This produces steel with superior impact resistance. Modern punches and chisels are often austempered. Because austempering does not produce martensite, the steel does not require further tempering.
A thorough discussion of tempering processes
{{Authority control Metal heat treatments
erosion
Erosion is the action of surface processes (such as water flow or wind) that removes soil, rock, or dissolved material from one location on the Earth's crust, and then transports it to another location where it is deposited. Erosion is d ...
, ablation
Ablation ( la, ablatio – removal) is removal or destruction of something from an object by vaporization, chipping, erosive processes or by other means. Examples of ablative materials are described below, and include spacecraft material for ...
, spalling
Spall are fragments of a material that are broken off a larger solid body. It can be produced by a variety of mechanisms, including as a result of projectile impact, corrosion, weathering, cavitation, or excessive rolling pressure (as in a ba ...
, or galling
Galling is a form of wear caused by adhesion between sliding surfaces. When a material galls, some of it is pulled with the contacting surface, especially if there is a large amount of force compressing the surfaces together. Galling is cau ...
.
* Structural integrity – The ability to withstand a maximum-rated load while resisting fracture, resisting fatigue, and producing a minimal amount of flexing or deflection
Deflection or deflexion may refer to:
Board games
* Deflection (chess), a tactic that forces an opposing chess piece to leave a square
* Khet (game), formerly ''Deflexion'', an Egyptian-themed chess-like game using lasers
Mechanics
* Deflection ...
, to provide a maximum service life
A product's service life is its period of use in service. Several related terms describe more precisely a product's life, from the point of manufacture, storage, and distribution, and eventual use.
Service life has been defined as "a product's ...
.
Carbon steel
Very few metals react to heat treatment in the same manner, or to the same extent, thatcarbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, coba ...
does, and carbon-steel heat-treating behavior can vary radically depending on alloying elements. Steel can be softened to a very malleable state through annealing, or it can be hardened to a state as hard and brittle as glass by quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as ...
. However, in its hardened state, steel is usually far too brittle, lacking the fracture toughness
In materials science, fracture toughness is the critical stress intensity factor of a sharp crack where propagation of the crack suddenly becomes rapid and unlimited. A component's thickness affects the constraint conditions at the tip of a ...
to be useful for most applications. Tempering is a method used to decrease the hardness, thereby increasing the ductility of the quenched steel, to impart some springiness and malleability to the metal. This allows the metal to bend before breaking. Depending on how much temper is imparted to the steel, it may bend elastically (the steel returns to its original shape once the load is removed), or it may bend plastically (the steel does not return to its original shape, resulting in permanent deformation), before fracturing. Tempering is used to precisely balance the mechanical properties of the metal, such as shear strength, yield strength
In materials science and engineering, the yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and wi ...
, hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
, ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
and tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials ...
, to achieve any number of a combination of properties, making the steel useful for a wide variety of applications. Tools such as hammers and wrenches require good resistance to abrasion, impact resistance, and resistance to deformation. Springs do not require as much wear resistance, but must deform elastically without breaking. Automotive parts tend to be a little less strong, but need to deform plastically before breaking.
Except in rare cases where maximum hardness or wear resistance is needed, such as the untempered steel used for files, quenched steel is almost always tempered to some degree. However, steel is sometimes annealed through a process called normalizing, leaving the steel only partially softened. Tempering is sometimes used on normalized steels to further soften it, increasing the malleability and machinability for easier metalworking
Metalworking is the process of shaping and reshaping metals to create useful objects, parts, assemblies, and large scale structures. As a term it covers a wide and diverse range of processes, skills, and tools for producing objects on every scale ...
. Tempering may also be used on welded steel, to relieve some of the stresses and excess hardness created in the heat affected zone
In fusion welding, the heat-affected zone (HAZ) is the area of base material, either a metal or a thermoplastic, which is not melted but has had its microstructure and properties altered by welding or heat intensive cutting operations. The heat ...
around the weld.
Quenched steel
Tempering is most often performed on steel that has been heated above its upper critical (A3) temperature and then quickly cooled, in a process calledquenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as ...
, using methods such as immersing the hot steel in water, oil, or forced-air. The quenched steel, being placed in or very near its hardest possible state, is then tempered to incrementally decrease the hardness to a point more suitable for the desired application. The hardness of the quenched steel depends on both cooling speed and on the composition of the alloy. Steel with a high carbon content will reach a much harder state than steel with a low carbon content. Likewise, tempering high-carbon steel to a certain temperature will produce steel that is considerably harder than low-carbon steel that is tempered at the same temperature. The amount of time held at the tempering temperature also has an effect. Tempering at a slightly elevated temperature for a shorter time may produce the same effect as tempering at a lower temperature for a longer time. Tempering times vary, depending on the carbon content, size, and desired application of the steel, but typically range from a few minutes to a few hours.
Tempering quenched steel at very low temperatures, between , will usually not have much effect other than a slight relief of some of the internal stresses and a decrease in brittleness. Tempering at higher temperatures, from , will produce a slight reduction in hardness, but will primarily relieve much of the internal stresses. In some steels with low alloy content, tempering in the range of causes a decrease in ductility and an increase in brittleness, and is referred to as the "tempered martensite embrittlement" (TME) range. Except in the case of blacksmithing, this range is usually avoided. Steel requiring more strength than toughness, such as tools, are usually not tempered above . Instead, a variation in hardness is usually produced by varying only the tempering time. When increased toughness is desired at the expense of strength, higher tempering temperatures, from , are used. Tempering at even higher temperatures, between , will produce excellent toughness, but at a serious reduction in the strength and hardness. At , the steel may experience another stage of embrittlement, called "temper embrittlement" (TE), which occurs if the steel is held within the temperature range of temper embrittlement for too long. When heating above this temperature, the steel will usually not be held for any amount of time, and quickly cooled to avoid temper embrittlement.
Normalized steel
Steel that has been heated above its upper critical temperature and then cooled in standing air is called normalized steel. Normalized steel consists of pearlite,martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation.
Properties
M ...
and sometimes bainite grains, mixed together within the microstructure. This produces steel that is much stronger than full-annealed steel, and much tougher than tempered quenched-steel. However, added toughness is sometimes needed at a reduction in strength. Tempering provides a way to carefully decrease the hardness of the steel, thereby increasing the toughness to a more desirable point. Cast-steel is often normalized rather than annealed, to decrease the amount of distortion that can occur. Tempering can further decrease the hardness, increasing the ductility to a point more like annealed steel. Tempering is often used on carbon steels, producing much the same results. The process, called "normalize and temper", is used frequently on steels such as 1045 carbon steel, or most other steels containing 0.35 to 0.55% carbon. These steels are usually tempered after normalizing, to increase the toughness and relieve internal stresses. This can make the metal more suitable for its intended use and easier to machine
A machine is a physical system using power to apply forces and control movement to perform an action. The term is commonly applied to artificial devices, such as those employing engines or motors, but also to natural biological macromolecul ...
.
Welded steel
Steel that has been arc welded, gas welded, or welded in any other manner besides forge welded, is affected in a localized area by the heat from the welding process. This localized area, called theheat-affected zone
In fusion welding, the heat-affected zone (HAZ) is the area of base material, either a metal or a thermoplastic, which is not melted but has had its microstructure and properties altered by welding or heat intensive cutting operations. The heat ...
(HAZ), consists of steel that varies considerably in hardness, from normalized steel to steel nearly as hard as quenched steel near the edge of this heat-affected zone. Thermal contraction from the uneven heating, solidification and cooling creates internal stresses in the metal, both within and surrounding the weld. Tempering is sometimes used in place of stress relieving (even heating and cooling of the entire object to just below the A1 temperature) to both reduce the internal stresses and to decrease the brittleness around the weld. Localized tempering is often used on welds when the construction is too large, intricate, or otherwise too inconvenient to heat the entire object evenly. Tempering temperatures for this purpose are generally around and .
Quench and self-temper
Modern reinforcing bar of 500 MPa strength can be made from expensivemicroalloyed steel
Microalloyed steel is a type of alloy steel that contains small amounts of alloying elements (0.05 to 0.15%), including niobium, vanadium, titanium, molybdenum, zirconium, boron, and rare-earth metals. They are used to refine the grain microstructu ...
or by a quench and self-temper (QST) process. After the bar exits the final rolling pass, where the final shape of the bar is applied, the bar is then sprayed with water which quenches the outer surface of the bar. The bar speed and the amount of water are carefully controlled in order to leave the core of the bar unquenched. The hot core then tempers the already quenched outer part, leaving a bar with high strength but with a certain degree of ductility too.
Blacksmithing
Tempering was originally a process used and developed by blacksmiths (forgers of iron). The process was most likely developed by theHittites
The Hittites () were an Anatolian people who played an important role in establishing first a kingdom in Kussara (before 1750 BC), then the Kanesh or Nesha kingdom (c. 1750–1650 BC), and next an empire centered on Hattusa in north-cent ...
of Anatolia
Anatolia, tr, Anadolu Yarımadası), and the Anatolian plateau, also known as Asia Minor, is a large peninsula in Western Asia and the westernmost protrusion of the Asian continent. It constitutes the major part of modern-day Turkey. The re ...
(modern-day Turkey), in the twelfth or eleventh century BC. Without knowledge of metallurgy, tempering was originally devised through a trial-and-error method.
Because few methods of precisely measuring temperature existed until modern times, temperature was usually judged by watching the tempering colors of the metal. Tempering often consisted of heating above a charcoal or coal forge, or by fire, so holding the work at exactly the right temperature for the correct amount of time was usually not possible. Tempering was usually performed by slowly, evenly overheating the metal, as judged by the color, and then immediately cooling, either in open air or by immersing in water. This produced much the same effect as heating at the proper temperature for the right amount of time, and avoided embrittlement by tempering within a short time period. However, although tempering-color guides exist, this method of tempering usually requires a good amount of practice to perfect, because the final outcome depends on many factors, including the composition of the steel, the speed at which it was heated, the type of heat source ( oxidizing or carburizing), the cooling rate, oil films or impurities on the surface, and many other circumstances which vary from smith to smith or even from job to job. The thickness of the steel also plays a role. With thicker items, it becomes easier to heat only the surface to the right temperature, before the heat can penetrate through. However, very thick items may not be able to harden all the way through during quenching.
Tempering colors
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
layer on its surface when heated. As the temperature of the steel is increased, the thickness of the iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of wh ...
will also increase. Although iron oxide is not normally transparent, such thin layers do allow light to pass through, reflecting off both the upper and lower surfaces of the layer. This causes a phenomenon called thin-film interference, which produces colors on the surface. As the thickness of this layer increases with temperature, it causes the colors to change from a very light yellow, to brown, then purple, then blue. These colors appear at very precise temperatures, and provide the blacksmith with a very accurate gauge for measuring the temperature. The various colors, their corresponding temperatures, and some of their uses are:
* Faint-yellow – – gravers, razors, scrapers
* Light-straw – – rock drills, reamers, metal-cutting saws
* Dark-straw – – scribers, planer blades
* Brown – – taps, dies, drill bits, hammers, cold chisels
* Purple – – surgical tools, punches, stone carving tools
* Dark blue – – screwdrivers, wrenches
* Light blue – – springs, wood-cutting saws
* Grey-blue – and higher – structural steel
Beyond the grey-blue color, the iron oxide loses its transparency, and the temperature can no longer be judged in this way. The layer will also increase in thickness as time passes, which is another reason overheating and immediate cooling is used. Steel in a tempering oven, held at for a long time, will begin to turn brown, purple or blue, even though the temperature did not exceed that needed to produce a light-straw color. Oxidizing or carburizing heat sources may also affect the final result. The iron oxide layer, unlike rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO( ...
, also protects the steel from corrosion through passivation.
Differential tempering
 Differential tempering is a method of providing different amounts of temper to different parts of the steel. The method is often used in bladesmithing, for making knives and
Differential tempering is a method of providing different amounts of temper to different parts of the steel. The method is often used in bladesmithing, for making knives and sword
A sword is an edged, bladed weapon intended for manual cutting or thrusting. Its blade, longer than a knife or dagger, is attached to a hilt and can be straight or curved. A thrusting sword tends to have a straighter blade with a pointed ti ...
s, to provide a very hard edge while softening the spine or center of the blade. This increased the toughness while maintaining a very hard, sharp, impact-resistant edge, helping to prevent breakage. This technique was more often found in Europe, as opposed to the differential hardening techniques more common in Asia, such as in Japanese swordsmithing.
Differential tempering consists of applying heat to only a portion of the blade, usually the spine, or the center of double-edged blades. For single-edged blades, the heat, often in the form of a flame or a red-hot bar, is applied to the spine of the blade only. The blade is then carefully watched as the tempering colors form and slowly creep toward the edge. The heat is then removed before the light-straw color reaches the edge. The colors will continue to move toward the edge for a short time after the heat is removed, so the smith typically removes the heat a little early, so that the pale-yellow just reaches the edge, and travels no farther. A similar method is used for double-edged blades, but the heat source is applied to the center of the blade, allowing the colors to creep out toward each edge.
Interrupted quenching
Interrupted quenching methods are often referred to as tempering, although the processes are very different from traditional tempering. These methods consist of quenching to a specific temperature that is above the martensite start (Ms) temperature, and then holding at that temperature for extended amounts of time. Depending on the temperature and the amount of time, this allows either pure bainite to form, or holds-off forming the martensite until much of the internal stresses relax. These methods are known as austempering and martempering.''Elements of metallurgy and engineering alloys'' By Flake C. Campbell - ASM International 2008 Page 195-196Austempering
 Austempering is a technique used to form pure bainite, a transitional microstructure found between pearlite and martensite. In normalizing, both upper and lower bainite are usually found mixed with pearlite. To avoid the formation of pearlite or martensite, the steel is quenched in a bath of molten metals or salts. This quickly cools the steel past the point where pearlite can form, and into the bainite-forming range. The steel is then held at the bainite-forming temperature, beyond the point where the temperature reaches an equilibrium, until the bainite fully forms. The steel is then removed from the bath and allowed to air-cool, without the formation of either pearlite or martensite.
Depending on the holding-temperature, austempering can produce either upper or lower bainite. Upper bainite is a laminate structure formed at temperatures typically above and is a much tougher microstructure. Lower bainite is a needle-like structure, produced at temperatures below 350 °C, and is stronger but much more brittle. In either case, austempering produces greater strength and toughness for a given hardness, which is determined mostly by composition rather than cooling speed, and reduced internal stresses which could lead to breakage. This produces steel with superior impact resistance. Modern punches and chisels are often austempered. Because austempering does not produce martensite, the steel does not require further tempering.
Austempering is a technique used to form pure bainite, a transitional microstructure found between pearlite and martensite. In normalizing, both upper and lower bainite are usually found mixed with pearlite. To avoid the formation of pearlite or martensite, the steel is quenched in a bath of molten metals or salts. This quickly cools the steel past the point where pearlite can form, and into the bainite-forming range. The steel is then held at the bainite-forming temperature, beyond the point where the temperature reaches an equilibrium, until the bainite fully forms. The steel is then removed from the bath and allowed to air-cool, without the formation of either pearlite or martensite.
Depending on the holding-temperature, austempering can produce either upper or lower bainite. Upper bainite is a laminate structure formed at temperatures typically above and is a much tougher microstructure. Lower bainite is a needle-like structure, produced at temperatures below 350 °C, and is stronger but much more brittle. In either case, austempering produces greater strength and toughness for a given hardness, which is determined mostly by composition rather than cooling speed, and reduced internal stresses which could lead to breakage. This produces steel with superior impact resistance. Modern punches and chisels are often austempered. Because austempering does not produce martensite, the steel does not require further tempering.
Martempering
Martempering is similar to austempering, in that the steel is quenched in a bath of molten metal or salts to quickly cool it past the pearlite-forming range. However, in martempering, the goal is to create martensite rather than bainite. The steel is quenched to a much lower temperature than is used for austempering; to just above the martensite start temperature. The metal is then held at this temperature until the temperature of the steel reaches an equilibrium. The steel is then removed from the bath before any bainite can form, and then is allowed to air-cool, turning it into martensite. The interruption in cooling allows much of the internal stresses to relax before the martensite forms, decreasing the brittleness of the steel. However, the martempered steel will usually need to undergo further tempering to adjust the hardness and toughness, except in rare cases where maximum hardness is needed but the accompanying brittleness is not. Modern files are often martempered.Physical processes
Tempering involves a three-step process in which unstable martensite decomposes into ferrite and unstable carbides, and finally into stable cementite, forming various stages of a microstructure called tempered martensite. The martensite typically consists of laths (strips) or plates, sometimes appearing acicular (needle-like) or lenticular (lens-shaped). Depending on the carbon content, it also contains a certain amount of "retained austenite." Retained austenite are crystals which are unable to transform into martensite, even after quenching below the martensite finish (Mf) temperature. An increase in alloying agents or carbon content causes an increase in retained austenite. Austenite has much higher stacking-fault energy than martensite or pearlite, lowering the wear resistance and increasing the chances ofgalling
Galling is a form of wear caused by adhesion between sliding surfaces. When a material galls, some of it is pulled with the contacting surface, especially if there is a large amount of force compressing the surfaces together. Galling is cau ...
, although some or most of the retained austenite can be transformed into martensite by cold and cryogenic treatments prior to tempering.
The martensite forms during a diffusionless transformation
A diffusionless transformation is a phase change that occurs without the long-range diffusion of atoms but rather by some form of cooperative, homogenous movement of many atoms that results in a change in the crystal structure. These movements ...
, in which the transformation occurs due to shear-stresses created in the crystal lattices rather than by chemical changes that occur during precipitation. The shear-stresses create many defects, or "dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to s ...
s," between the crystals, providing less-stressful areas for the carbon atoms to relocate. Upon heating, the carbon atoms first migrate to these defects, and then begin forming unstable carbides. This reduces the amount of total martensite by changing some of it to ferrite. Further heating reduces the martensite even more, transforming the unstable carbides into stable cementite.
The first stage of tempering occurs between room-temperature and . In the first stage, carbon precipitates into ε-carbon (Fe2,4C). In the second stage, occurring between and , the retained austenite transforms into a form of lower-bainite containing ε-carbon rather than cementite (archaically referred to as "troostite"). The third stage occurs at and higher. In the third stage, ε-carbon precipitates into cementite, and the carbon content in the martensite decreases. If tempered at higher temperatures, between and , or for longer amounts of time, the martensite may become fully ferritic and the cementite may become coarser or spheroidize. In spheroidized steel, the cementite network breaks apart and recedes into rods or spherical shaped globules, and the steel becomes softer than annealed steel; nearly as soft as pure iron, making it very easy to form
Form is the shape, visual appearance, or configuration of an object. In a wider sense, the form is the way something happens.
Form also refers to:
*Form (document), a document (printed or electronic) with spaces in which to write or enter data
* ...
or machine
A machine is a physical system using power to apply forces and control movement to perform an action. The term is commonly applied to artificial devices, such as those employing engines or motors, but also to natural biological macromolecul ...
.
Embrittlement
Embrittlement occurs during tempering when, through a specific temperature range, the steel experiences an increase in hardness and a reduction in ductility, as opposed to the normal decrease in hardness that occurs to either side of this range. The first type is called tempered martensite embrittlement (TME) or one-step embrittlement. The second is referred to as temper embrittlement (TE) or two-step embrittlement. One-step embrittlement usually occurs in carbon steel at temperatures between and , and was historically referred to as "500 degree ahrenheitembrittlement." This embrittlement occurs due to the precipitation of Widmanstatten needles or plates, made of cementite, in the interlath boundaries of the martensite. Impurities such asphosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, or alloying agents like manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
, may increase the embrittlement, or alter the temperature at which it occurs. This type of embrittlement is permanent, and can only be relieved by heating above the upper critical temperature and then quenching again. However, these microstructures usually require an hour or more to form, so are usually not a problem in the blacksmith-method of tempering.
Two-step embrittlement typically occurs by aging the metal within a critical temperature range, or by slowly cooling it through that range, For carbon steel, this is typically between and , although impurities like phosphorus and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
increase the effect dramatically. This generally occurs because the impurities are able to migrate to the grain boundaries, creating weak spots in the structure. The embrittlement can often be avoided by quickly cooling the metal after tempering. Two-step embrittlement, however, is reversible. The embrittlement can be eliminated by heating the steel above and then quickly cooling.
Alloy steels
Many elements are often alloyed with steel. The main purpose for alloying most elements with steel is to increase its hardenability and to decrease softening under temperature. Tool steels, for example, may have elements likechromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
or vanadium added to increase both toughness and strength, which is necessary for things like wrench
A wrench or spanner is a tool used to provide grip and mechanical advantage in applying torque to turn objects—usually rotary fasteners, such as nuts and bolts—or keep them from turning.
In the UK, Ireland, Australia, and New Zeal ...
es and screwdriver
A screwdriver is a tool, manual or powered, used for turning screws. A typical simple screwdriver has a handle and a shaft, ending in a tip the user puts into the screw head before turning the handle. This form of the screwdriver has been repla ...
s. On the other hand, drill bits and rotary file
Burrs or burs (sometimes called rotary files) are small cutting tools; not to be confused with small pieces of metal formed from cutting metal, used in die grinders, rotary tools, or dental drills. The name may be considered appropriate when the ...
s need to retain their hardness at high temperatures. Adding cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
or molybdenum can cause the steel to retain its hardness, even at red-hot temperatures, forming high-speed steels. Often, small amounts of many different elements are added to the steel to give the desired properties, rather than just adding one or two.
Most alloying elements (solutes) have the benefit of not only increasing hardness, but also lowering both the martensite start temperature and the temperature at which austenite transforms into ferrite and cementite. During quenching, this allows a slower cooling rate, which allows items with thicker cross-sections to be hardened to greater depths than is possible in plain carbon-steel, producing more uniformity in strength.
Tempering methods for alloy steels may vary considerably, depending on the type and amount of elements added. In general, elements like manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
, and aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
will remain dissolved in the ferrite during tempering while the carbon precipitates. When quenched, these solutes will usually produce an increase in hardness over plain carbon-steel of the same carbon content. When hardened alloy-steels, containing moderate amounts of these elements, are tempered, the alloy will usually soften somewhat proportionately to carbon steel.
However, during tempering, elements like chromium, vanadium, and molybdenum precipitate with the carbon. If the steel contains fairly low concentrations of these elements, the softening of the steel can be retarded until much higher temperatures are reached, when compared to those needed for tempering carbon steel. This allows the steel to maintain its hardness in high temperature or high friction applications. However, this also requires very high temperatures during tempering, to achieve a reduction in hardness. If the steel contains large amounts of these elements, tempering may produce an increase in hardness until a specific temperature is reached, at which point the hardness will begin to decrease. For instance, molybdenum steels will typically reach their highest hardness around whereas vanadium steels will harden fully when tempered to around . When very large amounts of solutes are added, alloy steels may behave like precipitation hardening alloys, which do not soften at all during tempering.''Steels: Microstructure and Properties: Microstructure and Properties By Harry Bhadeshia, Robert Honeycombe -- Elsevier 2006Page 191--207
Cast iron
Cast iron comes in many types, depending on the carbon-content. However, they are usually divided into grey and white cast iron, depending on the form that the carbides take. In grey cast iron, the carbon is mainly in the form ofgraphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
, but in white cast iron, the carbon is usually in the form of cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, ...
. Grey cast iron consists mainly of the microstructure called pearlite, mixed with graphite and sometimes ferrite. Grey cast iron is usually used as-cast, with its properties being determined by its composition.
White cast iron is composed mostly of a microstructure called ledeburite
In iron and steel metallurgy, ledeburite is a mixture of 4.3% carbon in iron and is a eutectic mixture of austenite and cementite. Ledeburite is not a type of steel as the carbon level is too high although it may occur as a separate constituent ...
mixed with pearlite. Ledeburite is very hard, making the cast iron very brittle. If the white cast iron has a hypoeutectic composition, it is usually tempered to produce malleable or ductile cast iron. Two methods of tempering are used, called "white tempering" and "black tempering." The purposes of both tempering methods is to cause the cementite within the ledeburite to decompose, increasing the ductility.''Physical metallurgy for engineers'' By Miklós Tisza - ASM International 2002 Page 348-350
White tempering
Malleable (porous) cast iron is manufactured by white tempering. White tempering is used to burn off excess carbon, by heating it for extended amounts of time in an oxidizing environment. The cast iron will usually be held at temperatures as high as for as long as 60 hours. The heating is followed by a slow cooling rate of around 10 °C (18 °F) per hour. The entire process may last 160 hours or more. This causes the cementite to decompose from the ledeburite, and then the carbon burns out through the surface of the metal, increasing the malleability of the cast iron.Black tempering
Ductile (non-porous) cast iron (often called "black iron") is produced by black tempering. Unlike white tempering, black tempering is done in aninert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to ...
environment, so that the decomposing carbon does not burn off. Instead, the decomposing carbon turns into a type of graphite called "temper graphite" or "flaky graphite," increasing the malleability of the metal. Tempering is usually performed at temperatures as high as for up to 20 hours. The tempering is followed by slow-cooling through the lower critical temperature, over a period that may last from 50 to over 100 hours.
Precipitation hardening alloys
Precipitation hardening alloys first came into use during the early 1900s. Most heat-treatable alloys fall into the category of precipitation hardening alloys, including alloys ofaluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
. Several high- alloy steels are also precipitation hardening alloys. These alloys become softer than normal when quenched, and then harden over time. For this reason, precipitation hardening is often referred to as "aging."
Although most precipitation hardening alloys will harden at room temperature, some will only harden at elevated temperatures and, in others, the process can be sped up by aging at elevated temperatures. Aging at temperatures higher than room-temperature is called "artificial aging". Although the method is similar to tempering, the term "tempering" is usually not used to describe artificial aging, because the physical processes, (i.e.: precipitation of intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
phases from a supersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a li ...
alloy) the desired results, (i.e.: strengthening rather than softening), and the amount of time held at a certain temperature are very different from tempering as used in carbon-steel.
See also
*Annealing (metallurgy)
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a mater ...
* Austempering
Austempering is heat treatment that is applied to ferrous metals, most notably steel and ductile iron. In steel it produces a bainite microstructure whereas in cast irons it produces a structure of acicular ferrite and high carbon, stabilized au ...
* Precipitation strengthening
Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, ...
* Tempered glass
References
Further reading
*Manufacturing Processes Reference Guide by Robert H. Todd, Dell K. Allen, and Leo Alting pg. 410External links
A thorough discussion of tempering processes
{{Authority control Metal heat treatments