TNNT3 on:
[Wikipedia]
[Google]
[Amazon]
Fast skeletal muscle troponin T (fTnT) is a
 TNNT3 gene evolved as one of the three TnT
TNNT3 gene evolved as one of the three TnT 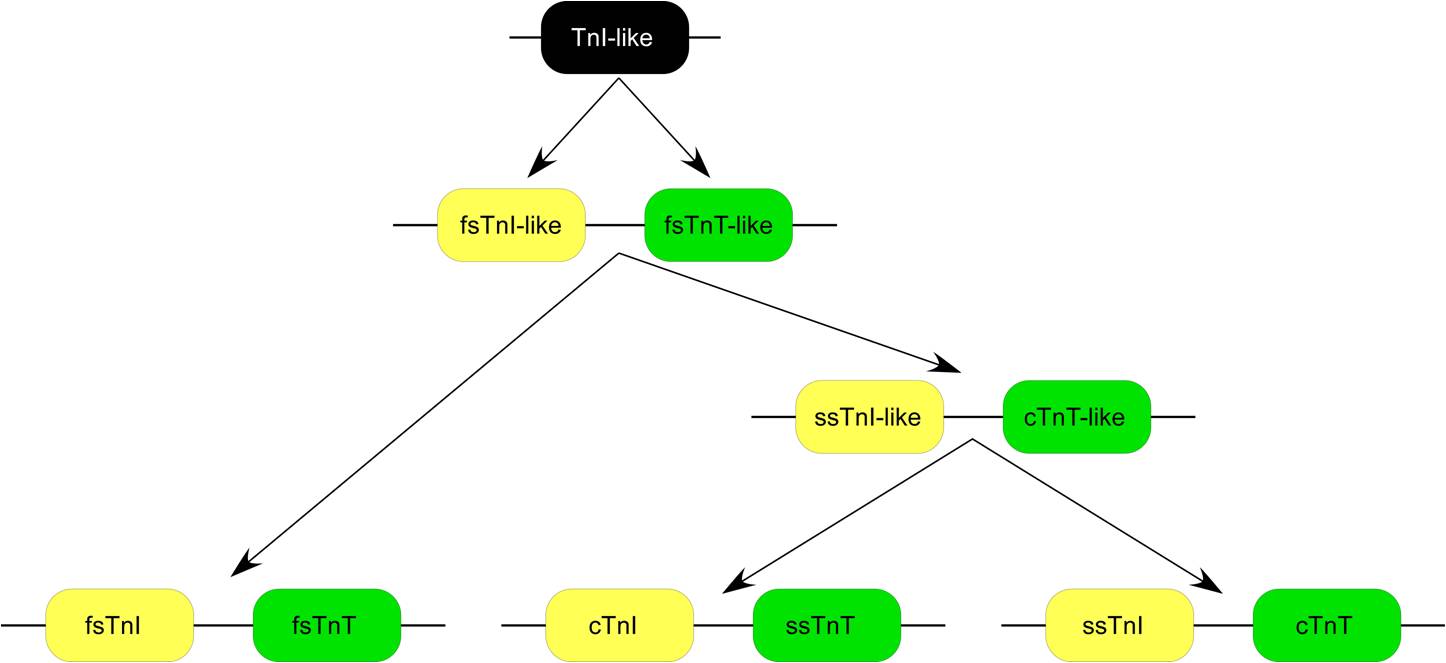
 Through alternative splicing of the fetal exon and other alternative exons in the N-terminal variable region, the expression of fsTnT during mammalian and avian development undergoes a high molecular to low molecular weight isoform switch in both fast and slow fiber dominant skeletal muscles. The inclusion of more N-terminal exons increases the negative charge that tunes the overall molecular conformation of fsTnT and alters interaction with TnI, TnC and tropomyosin. The alternative splicing-based addition of N-terminal negative charge in fsTnT also contributes to the tolerance to acidosis.
Alternative splicing of the two
Through alternative splicing of the fetal exon and other alternative exons in the N-terminal variable region, the expression of fsTnT during mammalian and avian development undergoes a high molecular to low molecular weight isoform switch in both fast and slow fiber dominant skeletal muscles. The inclusion of more N-terminal exons increases the negative charge that tunes the overall molecular conformation of fsTnT and alters interaction with TnI, TnC and tropomyosin. The alternative splicing-based addition of N-terminal negative charge in fsTnT also contributes to the tolerance to acidosis.
Alternative splicing of the two
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
that in humans is encoded by the ''TNNT3'' gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba ...
.
The TNNT3 gene is located at 11p15.5 in the human genome
The human genome is a complete set of nucleic acid sequences for humans, encoded as DNA within the 23 chromosome pairs in cell nuclei and in a small DNA molecule found within individual mitochondria. These are usually treated separately as the n ...
, encoding the fast skeletal muscle
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscl ...
isoform of troponin T (fsTnT). fsTnT is an ~31-kDa protein consisting of 268 amino acids including the first methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ro ...
with an isoelectric point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also u ...
(pI) of 6.21 (embryonic form). fsTnT is the tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons.
Tropomyosin and the actin skeleton
All organisms contain organelles that provide physical integrity to their cells. These type of organelles ar ...
-binding and thin filament anchoring subunit of the troponin complex
image:Troponin Ribbon Diagram.png, 400px, Ribbon representation of the human cardiac troponin core complex (52 kDa core) in the calcium-saturated form. Blue = troponin C; green = troponin I; magenta = troponin T.; ; rendered with PyMOL
Troponin, ...
in the sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called musc ...
s of fast twitch skeletal muscle. TNNT3 gene is specifically expressed in vertebrate fast twitch skeletal muscles.
Evolution
 TNNT3 gene evolved as one of the three TnT
TNNT3 gene evolved as one of the three TnT isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isof ...
genes in vertebrates. Each of the TnT isoform genes is linked to an upstream troponin I
Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to act ...
(TnI, one of the other two subunits of the troponin complex) isoform gene, and fsTnT is linked with fsTnI genes (Fig. 1). Sequence homology and protein epitope allosteric similarity data suggest that TnT gene was originated by duplication of a TnI-like ancestor gene and fsTnT was the first TnT emerged. Whereas significantly diverged from the slow skeletal muscle TnT (ssTnT encoded by TNNT1
Slow skeletal muscle troponin T (sTnT) is a protein that in humans is encoded by the ''TNNT1'' gene.
The TNNT1 gene is located at 19q13.4 in the human chromosomal genome, encoding the slow twitch skeletal muscle isoform of troponin T (ssTnT). ss ...
) and cardiac TnT (cTnT encoded by TNNT2
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the ''TNNT2'' gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle c ...
), Structure of fsTnT is conserved among vertebrate species (Fig. 2), reflecting specialized functional features of the different muscle fiber types.

Alternative splicing
Mammalian TNNT3 gene contains 19 exons. Alternative RNA splicing of 8 of them significantly increases structural variations of fsTnT. Two variable regions of the fsTnT protein are generated by alternative splicing (Fig. 3). In theN-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
region of fsTnT, exons 4, 5, 6, 7 and 8 are alternatively spliced in adult skeletal muscle cells. A fetal fsTnT exon located between exons 8 and 9 is specifically expressed in embryonic muscle (Briggs and Schachat 1993). Exons 16 and 17, previously designated as α and β exons, in the C-terminal region of fsTnT are alternatively spliced in a mutually exclusive manner.
Avian Tnnt3 gene has evolved with additional alternatively spliced exons, w, P1-7(x) and y, encoding the N-terminal variable region (Fig. 3). Reflecting the power of combined alternative splicing of multiple exons to generate fsTnT variants, two-dimensional gel electrophoresis detected more than 40 different fsTnT splice forms in chicken leg muscle.
Developmental regulation
 Through alternative splicing of the fetal exon and other alternative exons in the N-terminal variable region, the expression of fsTnT during mammalian and avian development undergoes a high molecular to low molecular weight isoform switch in both fast and slow fiber dominant skeletal muscles. The inclusion of more N-terminal exons increases the negative charge that tunes the overall molecular conformation of fsTnT and alters interaction with TnI, TnC and tropomyosin. The alternative splicing-based addition of N-terminal negative charge in fsTnT also contributes to the tolerance to acidosis.
Alternative splicing of the two
Through alternative splicing of the fetal exon and other alternative exons in the N-terminal variable region, the expression of fsTnT during mammalian and avian development undergoes a high molecular to low molecular weight isoform switch in both fast and slow fiber dominant skeletal muscles. The inclusion of more N-terminal exons increases the negative charge that tunes the overall molecular conformation of fsTnT and alters interaction with TnI, TnC and tropomyosin. The alternative splicing-based addition of N-terminal negative charge in fsTnT also contributes to the tolerance to acidosis.
Alternative splicing of the two C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
mutually exclusive exons 16 and 17 appears also regulated during development. Exon 17 with a sequence more similar to the counterpart segment in ssTnT and cTnT is predominantly expressed in embryonic and neonatal fsTnT. Exon 16 of fsTnT was only found in adult skeletal muscles. Exons 16 and 17 both encode a 14 amino acids peptide fragment residing in the α-helix interfacing with TnI and TnC. Protein interaction studies revealed that incorporation of exon 17 weakened binding of fsTnT to TnC and tropomyosin. Therefore, alternative splicing of exons 16 and 17 regulates the binding of fsTnT with TnI, possibly TnC, and thus tunes the function of the troponin complex and skeletal muscle contractility during development.
Avian Tnnt3 gene with additional alternatively spliced exons has unique expression pattern. The seven P exons are specifically expressed in pectoral muscles but not leg muscles. During post hatch development of the avian pectoral muscles, the segment encoded by the P exons (named Tx from the original annotation of the coding exons as an x exon) is up-regulated and included predominantly in fsTnT of adult pectoral muscles. Each P exon encodes a pentapeptide AHH(A/E)A. The Tx segment of adult fsTnT in avian orders of Galliformes and Craciformes contains 7-9 H(A/E)AAH repeats that possess high affinity binding to transition metal ions Cu(II), Ni(II), Zn(II) and Co(II). The Tx segment of chicken breast muscle fsTnT also a binding capacity for calcium, presumably serves as a calcium reservoir in avian fast pectoral muscles. Together with more N-terminal negative charges, this function may contribute to the higher calcium sensitivity of chicken breast muscle than that of leg muscle.
The switch of high to low molecular weight splice forms occurs in avian leg muscles during post hatching development similar to that in developing mammalian skeletal muscles. Early during post hatch development of chicken pectoral muscles, fsTnT also shows a high to low molecular weight switch. However, around 28 days after hatch, fsTnT with Tx segment spliced-in is rapidly up-regulated and becomes the major fsTnT splice form in adult pectoral muscles.
Deficiency of ssTnT did not affect the developmental switch of fsTnT splice forms in ssTnT-null mice, indicating that the developmental alternative splicing of the fsTnT pre-mRNA is regulated independent of skeletal muscle fiber type abnormality and adaptation.
Notes
References
{{Cytoskeletal Proteins