TNNT1 on:
[Wikipedia]
[Google]
[Amazon]
Slow skeletal muscle troponin T (sTnT) is a
 Three
Three 
GeneReviews/NCBI/NIH/UW entry on Nemaline Myopathy
{{Cytoskeletal Proteins
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
that in humans is encoded by the ''TNNT1'' gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
.
The TNNT1 gene is located at 19q13.4 in the human chromosomal genome, encoding the slow twitch skeletal muscle isoform of troponin T
Troponin T (shortened TnT or TropT) is a part of the troponin complex, which are proteins integral to the contraction of skeletal and heart muscles. They are expressed in skeletal and cardiac myocytes. Troponin T binds to tropomyosin and help ...
(ssTnT). ssTnT is an ~32-kDa protein consisting of 262 amino acids (including the first methionine) with an isoelectric point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also u ...
(pI) of 5.95. It is the tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons.
Tropomyosin and the actin skeleton
All organisms contain organelles that provide physical integrity to their cells. These type of organelles a ...
binding and thin filament anchoring subunit of the troponin complex in the sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called mus ...
s of slow twitch skeletal muscle fibers. TNNT1 gene is specifically expressed in slow skeletal muscle of vertebrates, with one exception that dry land toad (Bufo) cardiac muscle expresses ssTnT other than cardiac TnT.
Evolution
 Three
Three homologous gene
Sequence homology is the homology (biology), biological homology between DNA sequence, DNA, RNA sequence, RNA, or Protein primary structure, protein sequences, defined in terms of shared ancestry in the evolutionary history of life. Two segments ...
s have evolved in vertebrates, encoding three muscle type specific isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some iso ...
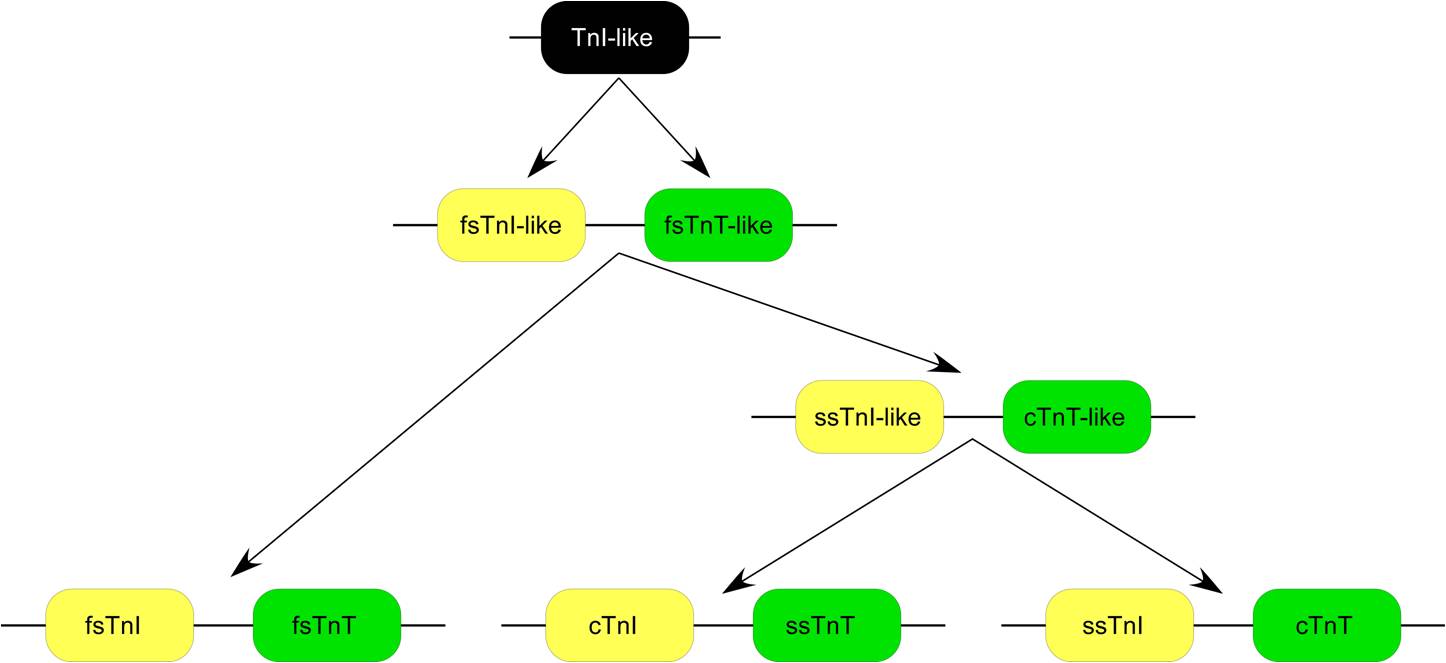
of TnT. Each of the TnT isoform genes is linked to one of the three troponin I isoform genes encoding the inhibitory subunit of the troponin complex, in chromosomal DNA to form three gene pairs: The fast skeletal muscle TnI (fsTnI)-fsTnT, ssTnI-cardiac (cTnT) and cTnI-ssTnT gene pairs. Sequence and epitope conservation studies suggested that genes encoding the muscle type specific TnT and TnI isoforms may have evolved from duplications of a fsTnI-like-fsTnT-like gene pair. Evolutionary lineage of the three TnI-TnT gene pairs shows that cTnI-ssTnT is the newest and most closely linked.
Protein sequence alignment demonstrated that TNNT1 genes are highly conserved among vertebrate species (Fig. 2), especially in the middle and C-terminal regions, while the three muscle type isoforms are significantly diverged among vertebrate species.

Alternative splicing
In mammalian and avian species, TNNT1 gene has a total of 14 exons, among which exon 5 encoding an 11-amino acid in the N-terminal region is alternatively spliced, generating a high molecular weight and a low molecular weight slow TnT splice forms (Jin, Chen et al. 1998). Biochemical studies showed that TnT splice forms have detectable different molecular conformation in the middle and C-terminal regions, producing different binding affinities for TnI and tropomyosin. The alternative splice forms of ssTnT play a role in skeletal muscle adaptation in physiologic and pathological conditions. Alternative splicing at alternative acceptor sites of intron 5 generates a single amino acid difference in the N-terminal region of ssTnT, of which functional significance has not been established.Clinical significance
A nonsense mutation E180X in the exon 11 of TNNT1 gene causes Amish Nemaline Myopathy (ANM), which is a severe form of recessive nemaline myopathy originally found in the Old Order Amish population in Pennsylvania, USA. Truncation of the ssTnT polypeptide chain by the E180X mutation deletes the tropomyosin-binding site 2 as well as the binding sites for TnI and troponin C (TnC) in the C-terminal region (Fig. 3). Consistent with the recessive phenotype, the truncated ssTnT is incapable of incorporation into the myofilaments and completely degraded in muscle cells. Tnnt1 gene targeted mouse studies reproduced the myopathic phenotypes of ANM. ssTnT null mice showed significantly decreased type I slow fibers in diaphragm and soleus muscles with hypertrophy of type II fast fibers, increased fatigability, and active regeneration of slow fibers (Wei, Lu et al. 2014). Recent case reports identified three more mutations in TNNT1 gene to cause nemaline myopathies outside the Amish population. A nonsense mutation S108X in exon 9 was identified in a Hispanic male patient with severe recessive nemaline myopathy phenotype. A Dutch patient with compound heterozygous TNNT1 gene mutations that cause exon 8 and exon 14 deletions also presents nemaline myopathy phenotypes. A rearrangement in TNNT1 gene (c.574_577 delins TAGTGCTGT) leading to aberrant splicing that causes C-terminal truncation of the protein (L203 truncation) was reported in 9 Palestinian patients from 7 unrelated families with recessively inherited nemaline Myopathy. Illustrated in Fig. 3, the S108X mutation truncates ssTnT protein to cause a loss of functional structures equivalent to that of E180X. The exon 8 deletion destructs the middle region tropomyosin-binding site 1. The L203 truncation deletes the binding sites for TnI and TnC but preserves both tropomyosin-binding sites 1 and 2. It remains to be investigated whether this novel mutation is able to bind the actin-tropomyosin thin filament in vivo and how it causes recessive nemaline myopathy. Alternative splicing of exon 5 produces high and low molecular weight splice forms of ssTnT. The low molecular ssTnT was significantly upregulated in type 1 (demyelination) but not type 2 (axon loss) Charcot-Marie-Tooth disease, suggesting that structural modification of TnT in the myofilaments may contribute to adaptation to abnormalities in neuronal activation.Interactions
TNNT1 has been shown to interact with PRKG1.Notes
References
External links
GeneReviews/NCBI/NIH/UW entry on Nemaline Myopathy
{{Cytoskeletal Proteins