Strong acids on:
[Wikipedia]
[Google]
[Amazon]
Acid strength is the tendency of an HA , to dissociate into a H+ , and an A- . The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.
:HA -> H+ + A-
Examples of (HCl) , (HClO4) , (HNO3) and (H2SO4) .
A weak acid is only partially dissociated, with both the undissociated acid and its dissociation products being present, in solution, in equilibrium with each other.
:HA <=> H+ + A-
CH3COOH ) is an example of a weak acid. The strength of a weak acid is quantified by its
H+ . Two key factors that contribute to the ease of H-A bond and the size of atom A, which determine the strength of the H-A bond. Acid strengths also depend on the stability of the conjugate base.
While the value measures the tendency of an acidic solute to transfer a proton to a standard solvent (most commonly water or DMSO), the tendency of an acidic solvent to transfer a proton to a reference solute (most commonly a weak H+ in solution. The pH of a simple solution of an acid in water is determined by both and the acid concentration. For weak acid solutions, it depends on the degree of dissociation, which may be determined by an equilibrium calculation. For concentrated solutions of acids, especially strong acids for which pH < 0, the value is a better measure of acidity than the pH.
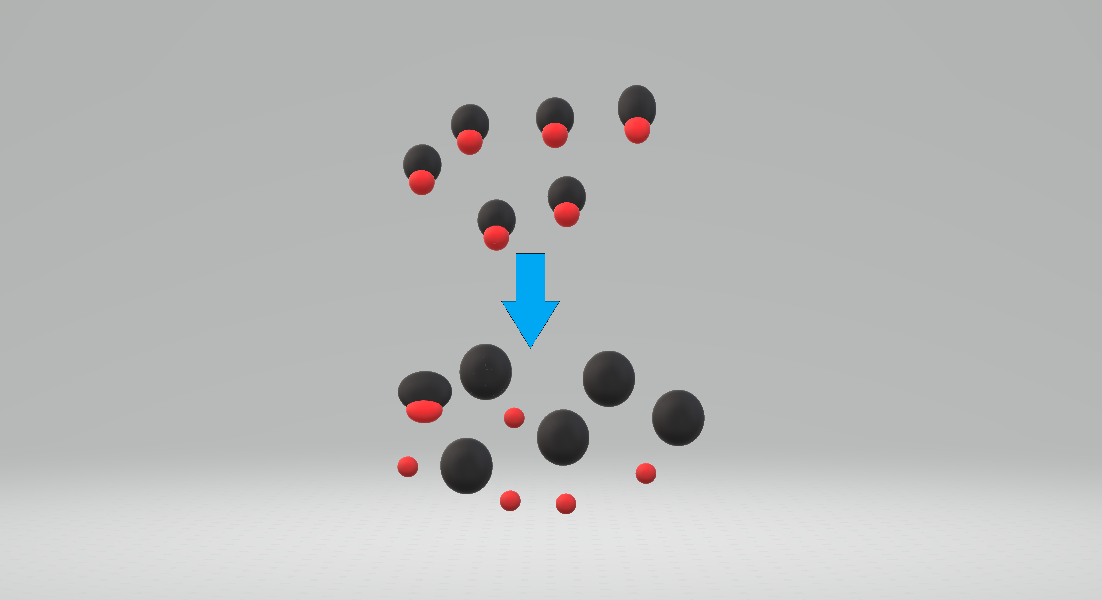 A ''strong acid'' is an acid that dissociates according to the reaction
:
A ''strong acid'' is an acid that dissociates according to the reaction
:HA + S <=> SH+ + A-
where S represents a solvent molecule, such as a molecule of water or HA is too low to be measured. For practical purposes a strong acid can be said to be completely dissociated. An example of a strong acid is HCl -> H+ + Cl- (in aqueous solution)
Any acid with a value which is less than about -2 is classed as a strong acid. This results from the very high HNO3 = −1.6
* H2SO4 (first dissociation only, ≈ −3)
The following can be used as protonators in H bF6/chem>
* H SO3SbF5/chem>
* Carborane superacid H HB11Cl11/chem>
* H
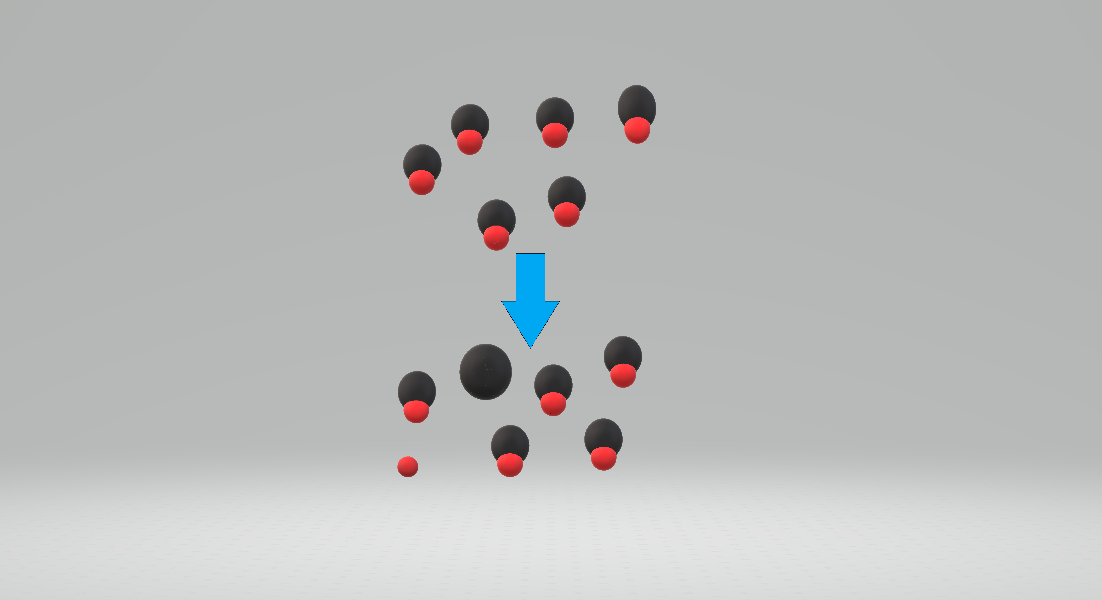 A weak acid is a substance that partially dissociates when it is dissolved in a solvent. In solution there is an equilibrium between the acid,
A weak acid is a substance that partially dissociates when it is dissolved in a solvent. In solution there is an equilibrium between the acid, HA , and the products of dissociation.
:
The solvent (e.g. water) is omitted from this expression when its concentration is effectively unchanged by the process of acid dissociation. The strength of a weak acid can be quantified in terms of a /chem>'' signifies the concentration of a chemical moiety, X.
:
When a numerical value of is known it can be used to determine the extent of dissociation in a solution with a given concentration of the acid, , by applying the law of /chem>.
:
This equation shows that the pH of a solution of a weak acid depends on both its value and its concentration. Typical examples of weak acids include HOOC-COOH ) is said to be dibasic because it can lose two protons and react with two molecules of a simple base. H3PO4 ) is tribasic.
For a more rigorous treatment of acid strength see
HA + OH- -> A- + H2O
until only the deprotonated species, A- , remains in solution. At each point in the titration pH is measured using a
HA + S<=> A- + HS+
For example, hydrochloric acid is a weak acid in solution in pure HO2CCH3 , which is more acidic than water.
:HO2CCH3 + HCl <=> (HO)2CCH3+ + Cl-
The extent of ionization of the HI > HBr > HCl . Acetic acid is said to be a differentiating solvent for the three acids, while water is not.
An important example of a solvent which is more basic than water is (CH3)2SO . A compound which is a weak acid in water may become a strong acid in DMSO.
Acidity–Basicity Data in Nonaqueous Solvents
- freeware for data analysis
and simulation of potentiometric titration curves Acids
acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
, symbolised by the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
, anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
, strong acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions ...
s are hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueo ...
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
Acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
(acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
, value.
The strength of a weak organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
acid may depend on substituent effects. The strength of an inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemist ...
acid is dependent on the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
for the atom to which the proton may be attached. Acid strength is solvent-dependent. For example, hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chlorid ...
is a strong acid in aqueous solution, but is a weak acid when dissolved in glacial acetic acid.
Measures of acid strength
The usual measure of the strength of an acid is itsacid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
(), which can be determined experimentally by titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
methods. Stronger acids have a larger and a smaller logarithmic constant () than weaker acids. The stronger an acid is, the more easily it loses a proton, deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
are the polarity of the aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
base) is measured by its Hammett acidity function
The Hammett acidity function (''H''0) is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity fu ...
, the value. Although these two concepts of acid strength often amount to the same general tendency of a substance to donate a proton, the and values are measures of distinct properties and may occasionally diverge. For instance, hydrogen fluoride, whether dissolved in water ( = 3.2) or DMSO ( = 15), has values indicating that it undergoes incomplete dissociation in these solvents, making it a weak acid. However, as the rigorously dried, neat acidic medium, hydrogen fluoride has an value of –15, making it a more strongly protonating medium than 100% sulfuric acid and thus, by definition, a superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
. (To prevent ambiguity, in the rest of this article, "strong acid" will, unless otherwise stated, refer to an acid that is strong as measured by its value ( < –1.74). This usage is consistent with the common parlance of most practicing chemists.)
When the acidic medium in question is a dilute aqueous solution, the is approximately equal to the pH value, which is a negative logarithm of the concentration of aqueous Strong acids
 A ''strong acid'' is an acid that dissociates according to the reaction
:
A ''strong acid'' is an acid that dissociates according to the reaction
:dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds a ...
(DMSO), to such an extent that the concentration of the undissociated species hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
:buffer capacity
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or Base (chemi ...
of solutions with a pH value
In chemistry, pH (), historically denoting "potential of hydrogen" (or "power of hydrogen"), is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions (solutions with higher concentrations of ions) are me ...
of 1 or less and is known as the leveling effect.
The following are strong acids in aqueous and dimethyl sulfoxide solution. The values of , cannot be measured experimentally. The values in the following table are average values from as many as 8 different theoretical calculations.
:
Also, in water
* Nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
Sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
* Fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric ...
Magic acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960s ...
Fluorosulfuric acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO ...
SO3 SO3 may refer to
*Sulfur trioxide, a chemical compound of sulfur and the anhydride of sulfuric acid
*Sulfite, a chemical ion composed of sulfur and oxygen with a 2− charge
* SO(3), the special orthogonal group in 3 dimensions; the rotations that ...
/chem>( = −6.4)
Sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is k ...
s, such as p-toluenesulfonic acid (tosylic acid) are a class of strong organic oxyacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produc ...
s. Some sulfonic acids can be isolated as solids. Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the Aromatic hydrocarbon, aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin pe ...
functionalized into polystyrene sulfonate is an example of a substance that is a solid strong acid.
Weak acids
 A weak acid is a substance that partially dissociates when it is dissolved in a solvent. In solution there is an equilibrium between the acid,
A weak acid is a substance that partially dissociates when it is dissolved in a solvent. In solution there is an equilibrium between the acid, dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex ...
, , defined as follows, where ''conservation of mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass can ...
.
:
where '''' is the value of the analytical concentration of the acid. When all the quantities in this equation are treated as numbers, ionic charges are not shown and this becomes a quadratic equation
In algebra, a quadratic equation () is any equation that can be rearranged in standard form as
ax^2 + bx + c = 0\,,
where represents an unknown value, and , , and represent known numbers, where . (If and then the equation is linear, not qu ...
in the value of the hydrogen ion concentration value, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
and phosphorous acid. An acid such as oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early invest ...
(Phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
(acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
. This includes acids such as the dibasic acid succinic acid
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological ro ...
, for which the simple method of calculating the pH of a solution, shown above, cannot be used.
Experimental determination
The experimental determination of a value is commonly performed by means of atitration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
. A typical procedure would be as follows. A quantity of strong acid is added to a solution containing the acid or a salt of the acid, to the point where the compound is fully protonated. The solution is then titrated with a strong base
:glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an exampl ...
and a pH meter
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH elect ...
. The equilibrium constant is found by fitting calculated pH values to the observed values, using the method of least squares
The method of least squares is a standard approach in regression analysis to approximate the solution of overdetermined systems (sets of equations in which there are more equations than unknowns) by minimizing the sum of the squares of the re ...
.
Conjugate acid/base pair
It is sometimes stated that "the conjugate of a weak acid is a strong base". Such a statement is incorrect. For example, acetic acid is a weak acid which has a = 1.75 x 10−5. Its conjugate base is theacetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
ion with ''K''b = 10−14/''K''a = 5.7 x 10−10 (from the relationship ''K''a × ''K''b = 10−14), which certainly does not correspond to a strong base. The conjugate of a weak acid is often a weak base and ''vice versa''.
Acids in non-aqueous solvents
The strength of an acid varies from solvent to solvent. An acid which is strong in water may be weak in a less basic solvent, and an acid which is weak in water may be strong in a more basic solvent. According toBrønsted–Lowry acid–base theory
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this theory ...
, the solvent S can accept a proton.
:acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
, hydrohalic acids
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. ...
decreases in the order dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds a ...
, DMSO, Acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
is an example of such a substance. An extensive bibliography of values in solution in DMSO and other solvents can be found aAcidity–Basicity Data in Nonaqueous Solvents
Superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
s are strong acids even in solvents of low dielectric constant. Examples of superacids are fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric ...
and magic acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960s ...
. Some superacids can be crystallised. They can also quantitatively stabilize carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
s.
Lewis acids reacting with Lewis bases in gas phase and non-aqueous solvents have been classified in the ECW model, and it has been shown that there is no one order of acid strengths. The relative acceptor strength of Lewis acids toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots. The plots shown in this paper used older parameters. Improved E&C parameters are listed in ECW model. It has been shown that to define the order of Lewis acid strength at least two properties must be considered. For the qualitative HSAB theory the two properties are hardness and strength while for the quantitative ECW model the two properties are electrostatic and covalent.
Factors determining acid strength
The inductive effect
In organic carboxylic acids, an electronegative substituent can pull electron density out of an acidic bond through the inductive effect, resulting in a smaller value. The effect decreases, the further the electronegative element is from the carboxylate group, as illustrated by the following series of halogenated butanoic acids.Effect of oxidation state
In a set of oxoacids of an element, values decrease with the oxidation state of the element. The oxoacids of chlorine illustrate this trend. † theoreticalReferences
{{reflist, 30emExternal links
* Titration of acid- freeware for data analysis
and simulation of potentiometric titration curves Acids