Steam methane reforming on:
[Wikipedia]
[Google]
[Amazon]
 Steam reforming or steam methane reforming (SMR) is a method for producing syngas (
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (CH4 + H2O <=> CO + 3 H2
The reaction is strongly
 The purpose of pre-reforming is to break down higher hydrocarbons such as propane, butane or naphta into
The purpose of pre-reforming is to break down higher hydrocarbons such as propane, butane or naphta into
 The reaction is conducted in multitubular packed bed reactors, a subtype of the plug flow reactor category. These reactors consist of an array of long and narrow tubes which are situated within the combustion chamber of a large
The reaction is conducted in multitubular packed bed reactors, a subtype of the plug flow reactor category. These reactors consist of an array of long and narrow tubes which are situated within the combustion chamber of a large
 Steam reforming or steam methane reforming (SMR) is a method for producing syngas (
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
) by reaction of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s with water. Commonly natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
is the feedstock. The main purpose of this technology is hydrogen production
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of ...
. The reaction is represented by this equilibrium:
:endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
(Δ''H''SR = 206 kJ/mol).
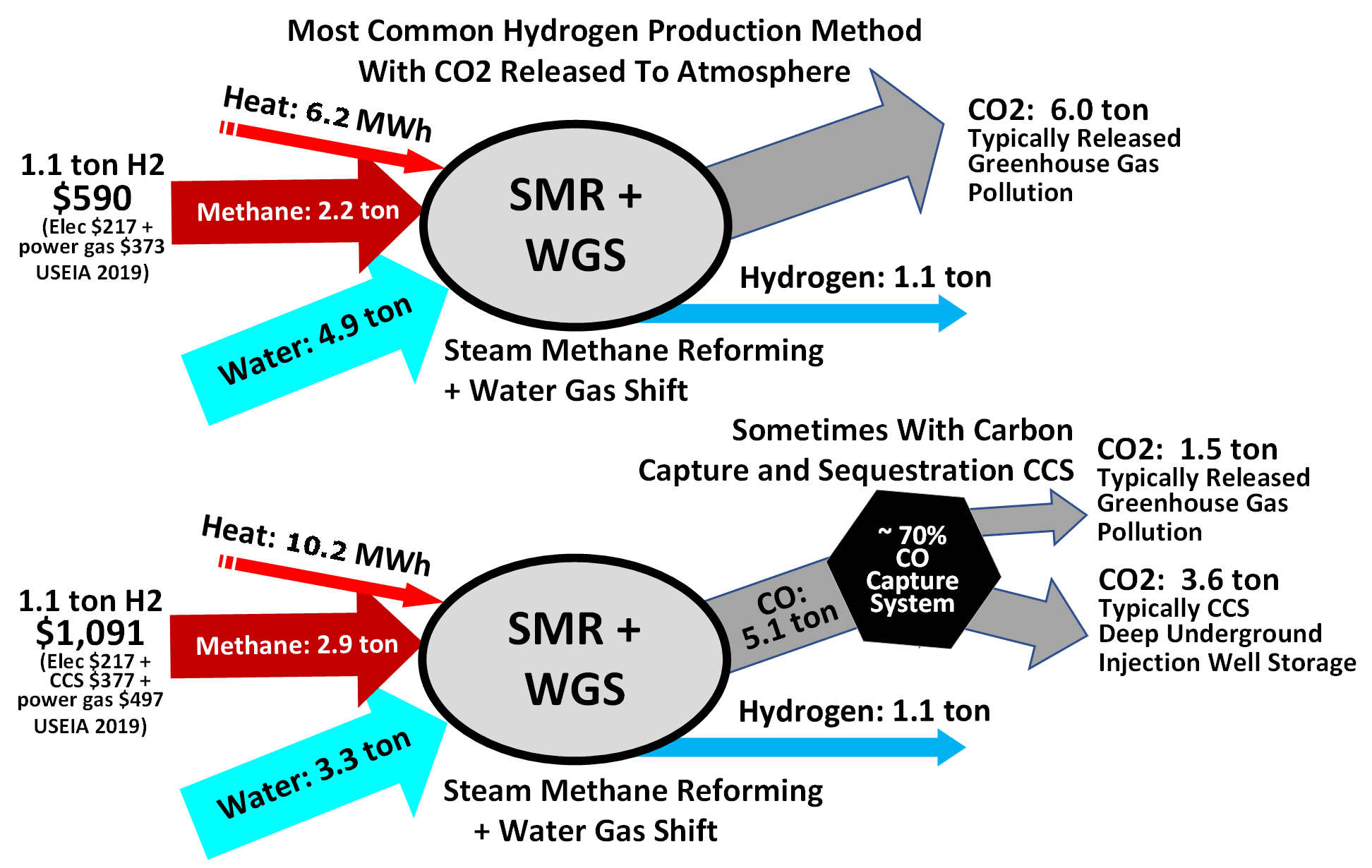
Hydrogen produced by steam reforming is termed 'grey hydrogen' when the waste carbon monoxide is released to the atmosphere and 'blue hydrogen' when the carbon monoxide is (mostly) captured and stored geologically - see carbon capture and storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually th ...
. Zero carbon 'green' hydrogen is produced by thermochemical water splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, base ...
, using solar thermal, low- or zero-carbon electricity or waste heat, or electrolysis, using low- or zero-carbon electricity. Zero carbon emissions 'turquoise' hydrogen is produced by one-step methane pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyr ...
of natural gas.
Steam reforming of natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
produces most of the world's hydrogen. Hydrogen is used in the industrial synthesis of ammonia and other chemicals.
Reactions
Steam reforming reaction kinetics, in particular usingnickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
- alumina catalysts, have been studied in detail since the 1950s.
Pre-reforming
 The purpose of pre-reforming is to break down higher hydrocarbons such as propane, butane or naphta into
The purpose of pre-reforming is to break down higher hydrocarbons such as propane, butane or naphta into methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
(CH4), which allows for more efficient reforming downstream.
Steam reforming
The name-giving reaction is the steam reforming (SR) reaction and is expressed by the equation: Via thewater-gas shift reaction
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer okewith air and gasifying it with steam". The caloric yield of this is about 10% of a modern syngas plant. F ...
(WGSR), additional hydrogen is released by reaction of water with the carbon monoxide generated according to equation
Some additional reactions occurring within steam reforming processes have been studied. Commonly the direct steam reforming (DSR) reaction is also included:
As these reactions by themselves are highly endothermic (apart from WGSR, which is mildly exothermic), a large amount of heat needs to be added to the reactor to keep a constant temperature. Optimal SMR reactor operating conditions lie within a temperature range of 800 °C to 900 °C at medium pressures of 20-30 bar. High excess of steam is required, expressed by the (molar) steam-to-carbon (S/C) ratio. Typical S/C ratio values lie within the range 2.5:1 - 3:1.
Industrial practice
 The reaction is conducted in multitubular packed bed reactors, a subtype of the plug flow reactor category. These reactors consist of an array of long and narrow tubes which are situated within the combustion chamber of a large
The reaction is conducted in multitubular packed bed reactors, a subtype of the plug flow reactor category. These reactors consist of an array of long and narrow tubes which are situated within the combustion chamber of a large industrial furnace
An industrial furnace, also known as a direct heater or a direct fired heater, is a device used to provide heat for an industrial process, typically higher than 400 degrees Celsius. They are used to provide heat for a process or can serve as r ...
, providing the necessary energy to keep the reactor at a constant temperature during operation. Furnace designs vary, depending on the burner configuration they are typically categorized into: top-fired, bottom-fired, and side-fired. A notable design is the Foster-Wheeler terrace wall reformer.
Inside the tubes, a mixture of steam and methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
are put into contact with a nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
catalyst. Catalysts with high surface-area-to-volume ratio
The surface-area-to-volume ratio, also called the surface-to-volume ratio and variously denoted sa/vol or SA:V, is the amount of surface area per unit volume of an object or collection of objects.
SA:V is an important concept in science and engin ...
are preferred because of diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
limitations due to high operating temperature. Examples of catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
shapes used are spoked wheels, gear wheels, and rings with holes (''see:'' ''Raschig rings''). Additionally, these shapes have a low pressure drop
Pressure drop is defined as the difference in total pressure between two points of a fluid carrying network. A pressure drop occurs when frictional forces, caused by the resistance to flow, act on a fluid as it flows through the tube. The main de ...
which is advantageous for this application.
Steam reforming of natural gas is 65–75% efficient.
The United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ...
produces 9–10 million tons of hydrogen per year, mostly with steam reforming of natural gas. The worldwide ammonia production, using hydrogen derived from steam reforming, was 144 million tonnes in 2018. The energy consumption has been reduced from 100 GJ/tonne of ammonia in 1920 to 27 GJ by 2019.
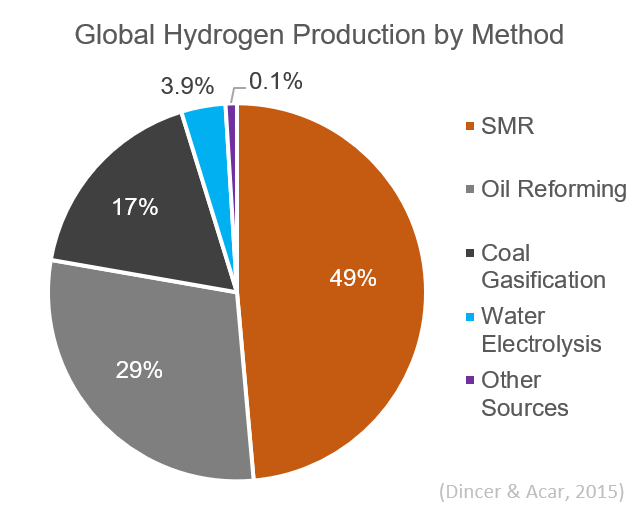
Globally, almost 50% of hydrogen is produced via steam reforming. It is currently the least expensive method for hydrogen production available in terms of its capital cost.
In an effort to decarbonise hydrogen production, carbon capture and storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually th ...
(CCS) methods are being implemented within the industry, which have the potential to remove up to 90% of CO2 produced from the process. Despite this, implementation of this technology remains problematic, costly, and increases the price of the produced hydrogen significantly.
Autothermal reforming
Autothermal reforming (ATR) uses oxygen and carbon dioxide or steam in a reaction with methane to form syngas. The reaction takes place in a single chamber where the methane is partially oxidized. The reaction is exothermic. When the ATR uses carbon dioxide, the H2:CO ratio produced is 1:1; when the ATR uses steam, the H2:CO ratio produced is 2.5:1. The outlet temperature of the syngas is between 950–1100 °C and outlet pressure can be as high as 100 bar. In addition to reactions – ATR introduces the following reaction: The main difference between SMR and ATR is that SMR only uses air for combustion as a heat source to create steam, while ATR uses purified oxygen. The advantage of ATR is that the H2:CO ratio can be varied, which can be useful for producing specialty products. Due to the exothermic nature of some of the additional reactions occurring within ATR, the process can essentially be performed at a net enthalpy of zero (Δ''H'' = 0).Partial oxidation
Partial oxidation (POX) occurs when a sub-stoichiometric fuel-air mixture is partially combusted in a reformer creating hydrogen-rich syngas. POX is typically much faster than steam reforming and requires a smaller reactor vessel. POX produces less hydrogen per unit of the input fuel than steam reforming of the same fuel.Steam reforming at small scale
The capital cost of steam reforming plants is considered prohibitive for small to medium size applications. The costs for these elaborate facilities do not scale down well. Conventional steam reforming plants operate at pressures between 200 and 600 psi (14–40 bar) with outlet temperatures in the range of 815 to 925 °C.For combustion engines
Flared gas and ventedvolatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a ...
s (VOCs) are known problems in the offshore industry and in the on-shore oil and gas industry, since both release greenhouse gases into the atmosphere. Reforming for combustion engines utilizes steam reforming technology for converting waste gases into a source of energy.
Reforming for combustion engines is based on steam reforming, where non-methane hydrocarbons (NMHC Non-methane volatile organic compounds (NMVOCs) are a set of organic compounds that are typically photochemically reactive in the atmosphere—marked by the exclusion of methane. NMVOCs include a large variety of chemically different compounds, suc ...
s) of low quality gases are converted to synthesis gas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used ...
(H2 + CO) and finally to methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
(CH4), carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
(CO2) and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
(H2) - thereby improving the fuel gas quality (methane number).
For fuel cells
There is also interest in the development of much smaller units based on similar technology to producehydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
as a feedstock for fuel cells. Small-scale steam reforming units to supply fuel cells are currently the subject of research and development, typically involving the reforming of methanol, but other fuels are also being considered such as propane, gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organi ...
, autogas
Autogas or LPG is liquefied petroleum gas (LPG) used as a fuel in internal combustion engines in vehicles as well as in stationary applications such as generators. It is a mixture of propane and butane.
Autogas is widely used as a "green" ...
, diesel fuel
Diesel fuel , also called diesel oil, is any liquid fuel specifically designed for use in a diesel engine, a type of internal combustion engine in which fuel ignition takes place without a spark as a result of compression of the inlet air and ...
, and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
.
Disadvantages
The reformer– the fuel-cell system is still being researched but in the near term, systems would continue to run on existing fuels, such as natural gas or gasoline or diesel. However, there is an active debate about whether using these fuels to make hydrogen is beneficial while global warming is an issue. Fossil fuel reforming does not eliminate carbon dioxide release into the atmosphere but reduces the carbon dioxide emissions and nearly eliminates carbon monoxide emissions as compared to the burning of conventional fuels due to increased efficiency and fuel cell characteristics. However, by turning the release of carbon dioxide into apoint source
A point source is a single identifiable ''localised'' source of something. A point source has negligible extent, distinguishing it from other source geometries. Sources are called point sources because in mathematical modeling, these sources ca ...
rather than distributed release, carbon capture and storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually th ...
becomes a possibility, which would prevent the carbon dioxide's release to the atmosphere, while adding to the cost of the process.
The cost of hydrogen production by reforming fossil fuels depends on the scale at which it is done, the capital cost of the reformer, and the efficiency of the unit, so that whilst it may cost only a few dollars per kilogram of hydrogen at an industrial scale, it could be more expensive at the smaller scale needed for fuel cells.
Challenges with reformers supplying fuel cells
There are several challenges associated with this technology: * The reforming reaction takes place at high temperatures, making it slow to start up and requiring costly high-temperature materials. * Sulfur compounds in the fuel will poison certain catalysts, making it difficult to run this type of system from ordinarygasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organi ...
. Some new technologies have overcome this challenge with sulfur-tolerant catalysts.
* Coking would be another cause of catalyst deactivation during steam reforming. High reaction temperatures, low steam-to-carbon ratio (S/C), and the complex nature of sulfur-containing commercial hydrocarbon fuels make coking especially favorable. Olefins, typically ethylene, and aromatics are well-known carbon-precursors, hence their formation must be reduced during steam reforming. Additionally, catalysts with lower acidity were reported to be less prone to coking by suppressing dehydrogenation reactions. H2S, the main product in the reforming of organic sulfur, can bind to all transition metal catalysts to form metal–sulfur bonds and subsequently reduce catalyst activity by inhibiting the chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
of reforming reactants. Meanwhile, the adsorbed sulfur species increases the catalyst acidity, and hence indirectly promotes coking. Precious metal catalysts such as Rh and Pt have lower tendencies to form bulk sulfides than other metal catalysts such as Ni. Rh and Pt are less prone to sulfur poisoning by only chemisorbing sulfur rather than forming metal sulfides.
* Low temperature polymer fuel cell membranes can be poisoned by the carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
(CO) produced by the reactor, making it necessary to include complex CO-removal systems. Solid oxide fuel cell
A solid oxide fuel cell (or SOFC) is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.
A ...
s (SOFC) and molten carbonate fuel cell
Molten-carbonate fuel cells (MCFCs) are high-temperature fuel cells that operate at temperatures of 600 °C and above.
Molten carbonate fuel cells (MCFCs) were developed for natural gas, biogas (produced as a result of anaerobic digestion or ...
s (MCFC) do not have this problem, but operate at higher temperatures, slowing start-up time, and requiring costly materials and bulky insulation.
* The thermodynamic efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For a ...
of the process is between 70% and 85% ( LHV basis) depending on the purity of the hydrogen product.
See also
*Biogas
Biogas is a mixture of gases, primarily consisting of methane, carbon dioxide and hydrogen sulphide, produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste and food waste. It is a ...
* Boudouard reaction
* Catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-oc ...
* Chemical looping reforming and gasification Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typi ...
* Cracking (chemistry)
* Hydrogen pinch
* Hydrogen technologies
Hydrogen technologies are technologies that relate to the production and use of hydrogen as a part hydrogen economy. Hydrogen technologies are applicable for many uses.
Some hydrogen technologies are carbon neutral and could have a role in preven ...
* Industrial gas
* Lane hydrogen producer
* Methane pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyr ...
(for Hydrogen)
* Partial oxidation Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A d ...
* PROX
PROX is an acronym for PReferential OXidation, and refers to the preferential oxidation of a carbon monoxide in a gas mixture by a catalyst. It is intended to remove trace amounts of CO from H2/CO/CO2 mixtures produced by steam reforming and wate ...
* Reformed methanol fuel cell
Reformed Methanol Fuel Cell (RMFC) or Indirect Methanol Fuel Cell (IMFC) systems are a subcategory of proton exchange membrane fuel cell, proton-exchange fuel cells where, the fuel, methanol (CH3OH), is reformed, before being fed into the fuel cel ...
* Reformer sponge iron cycle
* Syngas
* Timeline of hydrogen technologies
This is a timeline of the history of hydrogen technology.
Timeline
16th century
* c. 1520 – First recorded observation of hydrogen by Paracelsus through dissolution of metals (iron, zinc, and tin) in sulfuric acid.
17th century
* 1625 – F ...
References
{{DEFAULTSORT:Fossil Fuel Reforming Hydrogen production Chemical processes Fuel gas Catalysis Industrial gases