Spiro compound on:
[Wikipedia]
[Google]
[Amazon]
In
an
respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the

same access date. The Greek transcription, σπεῖρα, reflects the use of this cognate as one ancient Greek term to refer to a coil or related fold, see is a
same access date. * Examples of spiro natural products and their synthesis: * * The IUPAC documents on naming of spiro compounds: The full author (Working Party) list and a link to a German translation are provided in a corresponding footnote. ''Also available online at'' , same access date.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic
In chemistry, a bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all ...
(having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
that have no connecting atoms, from ''fused ring compounds'' like decalin
Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives.
I ...
having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan
respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the
acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
formed by reaction of a diol with a cyclic ketone. The common atom that connects the two (or sometimes three) rings is called the ''spiro atom''; in carbocyclic spiro compounds like spiro .5ndecane (see image at right), the spiro-atom is a quaternary carbon
A quaternary carbon is a carbon atom bound to four other carbon atoms. For this reason, quaternary carbon atoms are found only in hydrocarbons having at least five carbon atoms. Quaternary carbon atoms can occur in branched alkanes, but not in li ...
, and as the ''-ane'' ending implies, these are the types of molecules to which the name ''spirane'' was first applied (though it is now used general of all spiro compounds). Likewise, a tetravalent neutral silicon or positively charged quaternary nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atom ( ammonium cation) can be the spiro center in these compounds, and many of these have been prepared and described. The 2-3 rings being joined are most often different in nature, though they, on occasion, be identical .5ndecane,_just_shown,_and_spiropentadiene.html" ;"title=".g., spiro .5ndecane, just shown, and spiropentadiene">.g., spiro .5ndecane, just shown, and spiropentadiene, at right]. Although sketches of organic structures makes spiro compounds appear planar, they are not; for instance, a spiro compound with a pair of three-membered cyclopropene rings connected in spiro fashion (image below) has been given the popular misnomer of being a bow tie
The bow tie is a type of necktie. A modern bow tie is tied using a common shoelace knot, which is also called the bow knot for that reason. It consists of a ribbon of fabric tied around the collar of a shirt in a symmetrical manner so that t ...
structure, when it is not flat or planar like a bow tie. This can be stated another way, saying that the best-fit planes to each ring are often perpendicular or are otherwise non-coplanar to one another.
Spiro compounds are present throughout the natural world, some cases of which have been exploited to provide tool compounds for biomedical study and to serve as scaffolds for the design of therapeutic agents with novel shapes. As well, the spiro motif is present in various practical compound types (such as dyes), as well as in a wide variety of oligo- and polymeric materials designs, for the unique shapes and properties the spiro center imparts, e.g., in the design of electronically active materials in particular. In both cases, the presence of the spiro center, often with four distinct groups attached, and with its unique aspects of chirality, adds unique challenges to the chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In mod ...
of each compound type.
Carbocyclic spiro compounds
Bicyclic
In chemistry, a bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all ...
ring structures in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
that have two fully carbocyclic (all carbon) rings connected through just one atom are present both in natural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical sy ...
, as well as in esoteric targets of chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In mod ...
. The two carbocycles can be different in nature, or identical. In common targets derived from natural products, they are essentially always different. In esoteric targets, such as highly strained hydrocarbons like spiropentadiene
Spiropentadiene, or bowtiediene, is a hydrocarbon with formula C5H4. The simplest spiro-connected cycloalkene, it is very unstable—decomposing even below −100 °C—due to its high bond strain and does not occur in nature. I ...
, shown here, the rings can be identical. The atom connecting the two rings is called the ''spiro-atom''; in carbocyclic spiro compounds, the spiro-atom is a quaternary carbon
A quaternary carbon is a carbon atom bound to four other carbon atoms. For this reason, quaternary carbon atoms are found only in hydrocarbons having at least five carbon atoms. Quaternary carbon atoms can occur in branched alkanes, but not in li ...
. The 11-carbon bicyclic structure shown above, spiro .5ndecane, is also a fully carbocyclic spiro compound. While the presentation of this structure makes it appear fully planar, it is not. The best-fit planes to each six-atom ring above is near to perpendicular, and the best-fit planes to rings of spiro compounds are likewise generally non-coplanar. For instance, the structure of faux bow tie spiropentadiene
Spiropentadiene, or bowtiediene, is a hydrocarbon with formula C5H4. The simplest spiro-connected cycloalkene, it is very unstable—decomposing even below −100 °C—due to its high bond strain and does not occur in nature. I ...
, shown above, makes clear that the planes that are defined by the atoms of each ring—i.e., the best-fit plane of each cyclopropene—are orthogonal (perpendicular) to one another.
Heterocyclic spiro compounds
Spiro compounds are considered heterocyclic if the spiro atom or any atom in either ring are not carbon atoms. Cases include the presence of a spiro heteroatom such silicon and nitrogen (but also other Group IVA 4and other atom types) connecting the rings that have been observed or are under theoretical study; moreover, there are also many cases where one or more heteroatoms appear in one or more of the rings that are joined at a carbon spiro atom (e.g., where 1 oxygen spironolactones and 2 oxygen/2 sulfur ketals/thioketals are very common). A common case is the presence of two atoms that are not carbon in one of the rings, with those two rings both attached to the spiro atom; indeed, often the earliest exposure of a chemist in training to a spiro compound is to a heterocyclic form, the ketal (acetal) formed in theprotection
Protection is any measure taken to guard a thing against damage caused by outside forces. Protection can be provided to physical objects, including organisms, to systems, and to intangible things like civil and political rights. Although th ...
of ketones by diols and dithiol
In organic chemistry, a dithiol is a type of organosulfur compound with two thiol () functional groups. Their properties are generally similar to those of monothiols in terms of solubility, odor, and volatility. They can be classified according ...
s. An example of this is shown above, in the synthesis of the acetal 1,4-dioxaspiro .5ecane from cyclohexanone and ethanediol. In this case, because the four atoms attached to the spiro atom are not all carbons, the spiro atom is not a quaternary carbon. A further example of an acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
formed from a cyclic ketone, except with a dithiol
In organic chemistry, a dithiol is a type of organosulfur compound with two thiol () functional groups. Their properties are generally similar to those of monothiols in terms of solubility, odor, and volatility. They can be classified according ...
, is the spiro compound spirapril, which has a five-membered ring formed from 1,2-ethanedithiol
Ethane-1,2-dithiol, also known as EDT, is a colorless liquid with the formula C H( SH). It has a very characteristic odor which is compared by many people to rotten cabbage. It is a common building block in organic synthesis and an excellent liga ...
. Again, while the rings could be identical, in the heterocyclic case they are, again, almost always non-identical. Once again, the best-fit planes to each ring are generally non-coplanar to one another (i.e., the rings are not coplanar, despite appearing so in images).
Polyspiro compounds
A polyspiro compound is connected by two or more spiroatoms making up three or morerings
Ring may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
:(hence) to initiate a telephone connection
Arts, entertainment and media Film and ...
.
Nomenclature
Nomenclature
Nomenclature (, ) is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. The principles of naming vary from the relatively informal conventions of everyday speech to the internationally ag ...
for spiro compounds was first discussed by Adolf von Baeyer in 1900. The prefix ''spiro'' denotes two rings with a spiro junction. The main method of systematic nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
The ...
is to follow with square brackets containing the number of atoms in the smaller ring then the number of atoms in the larger ring, separated by a period, in each case excluding the spiroatom (the atom by which the two rings are bonded) itself. Position-numbering starts with an atom of the smaller ring adjacent to the spiroatom around the atoms of that ring, then the spiroatom itself, then around the atoms of the larger ring. For example, compound A in the image is called ''1-bromo-3-chlorospiro .5ecan-7-ol'', and compound B is called ''1-bromo-3-chlorospiro .6ecan-7-ol''.
Chirality
Spiranes can bechiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
, in three distinct ways. First, while nevertheless appearing to be twisted, they yet may have a chiral center making them analogous to any simple chiral compound, and second, while again appearing twisted, the specific location of substituents, as with alkylidenecycloalkanes, may make a spiro compound display ''central chirality'' (rather than axial chirality resulting from the twist); third, the substituents of the rings of the spiro compound may be such that the only reason they are chiral arises solely from the twist of their rings, e.g., in the simplest bicyclic case, where two structurally identical rings are attached via their spiro atom, resulting in a twisted presentation of the two rings. Hence, in the third case, the lack of planarity described above gives rise to what is termed axial chirality in otherwise identical isomeric pair of spiro compounds, because they differ only in the right- ''versus'' left-handed "twist" of structurally identical rings (as seen in allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which ...
s, sterically hindered biaryls, and alkylidenecycloalkanes as well). Assignment of absolute configuration of spiro compounds has been challenging, but a number of each type have been unequivocally assigned.
Some spiro compounds exhibit axial chirality. Spiroatoms can be the origin of chirality even when they lack the required four different substituents normally observed in chirality. When two rings are identical the priority is determined by a slight modification of the CIP system assigning a higher priority to one ring extension and a lower priority to an extension in the other ring. When rings are dissimilar the regular rules apply.
Preparation
Spiro compounds present unique preparative challenges, whether each ring contributing to its structure is unique or identical, or whether they are carbocyclic or heterocyclic—owing to the practical implications of tetra-functionalizing the central spiro atom (often with four different groups), and of the unique aspects of chirality that apply to these compounds.Specific methods
Some spiro compounds can be synthesized using the Pinacol-pinacolone rearrangement; for example, spiro .5ecane (final compound in following two line scheme) can be synthesized from symmetric 1,2-diols of the sort shown below .g., this route's starting material, (1,1′-bicyclopentyl)-1,1′-diol Initially, one of the carbinol moieties is protonated, allowing water toleave
Leave may refer to:
* Permission (disambiguation)
** Permitted absence from work
*** Leave of absence, a period of time that one is to be away from one's primary job while maintaining the status of employee
*** Annual leave, allowance of time away ...
, and yielding the corresponding carbocation (second structure, first row); this intermediate then undergoes a bond migration, resulting in ring expansion of the adjacent ring, with deprotionation unmasking the ketone functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
to complete the first line of the mechanism. This first product, a spirobicyclic ketone, is a spiro compound in its own right, and yields the further spiro carbinol and the alicyclic spiro hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
after two further reduction reaction
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
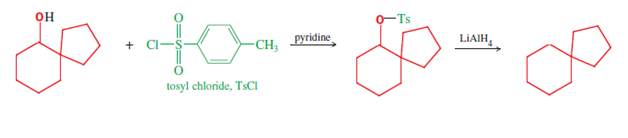
s. First, reduction of the carbonyl that ends the mechanism's first line provides the spiro carbinol starting material of the second line, which is needed for reduction to the alkane (shown). This latter reduction is accomplished using lithium aluminum hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic sy ...
(LiAlH4), via the alcohol tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on s ...
(formed using tosyl chloride). Hence this three reaction sequence provides three spiro compounds (ketone, alcohol, and alkane), of possible research or practical use.


Uses
Spiro forms oflactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
s and oxazines are frequently used as leuco dyes, frequently displaying chromism In chemistry, chromism is a process that induces a change, often reversible, in the colors of compounds. In most cases, chromism is based on a change in the electron states of molecules, especially the π- or d-electron state, so this phenomenon is ...
—reversible structural change between forms giving rise to colorless and colored appearances, especially in solution.
Spiroaromaticity
Spiroaromaticity inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
refers to a special case of aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
in which conjugation is interrupted by a single spiroatom. Although this spiro center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds.
Etymology
A spiro compound, or spirane, from the Latin ''spīra'', meaning a twist or coil, For a further but less stable source of the same text that provides access to the relevant material, sesame access date. The Greek transcription, σπεῖρα, reflects the use of this cognate as one ancient Greek term to refer to a coil or related fold, see is a
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
, typically an organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
, that presents a twisted structure of two or more rings (a ring system), in which 2 or 3 rings are linked together by one common atom, Note, the article co-authors, the Working Party of the IUPAC (1992-1998), were P. M. Giles, Jr., E. W. Godly, K.-H. Hellwich, A. K. Ikizler, M. V. Kisakürek, A. D. McNaught, G. P. Moss, J. Nyitrai, W. H. Powell, O. Weissbach, and A. Yerin. ''Also available online at'' ''Also available in German, with et al. indicating the same working party, at'' examples of which are shown at right.
Further reading
* * For a further but less stable source of the same text that provides access to the relevant material, sesame access date. * Examples of spiro natural products and their synthesis: * * The IUPAC documents on naming of spiro compounds: The full author (Working Party) list and a link to a German translation are provided in a corresponding footnote. ''Also available online at'' , same access date.
References
External links
{{Authority control Spiro compounds, *