Silicone rubber on:
[Wikipedia]
[Google]
[Amazon]
 Silicone rubber is an elastomer (rubber-like material) composed of
Silicone rubber is an elastomer (rubber-like material) composed of
 Two-part condensation systems package the cross-linker and condensation catalyst together in one part while the polymer and any fillers or pigments are in the second part. Mixing of the two parts causes the curing to take place. A typical filler is fumed silica, also known as pyrogenic silica, which used to control the flow properties of the sealant.
Once fully cured, condensation systems are effective as sealants and caulks in plumbing and building construction and as molds for casting polyurethane, epoxy and polyester resins, waxes, gypsum, and low-melting-temperature metals such as lead. They are typically very flexible and have a high tear strength. They do not require the use of a release agent since silicones have non-stick properties.
Two-part condensation systems package the cross-linker and condensation catalyst together in one part while the polymer and any fillers or pigments are in the second part. Mixing of the two parts causes the curing to take place. A typical filler is fumed silica, also known as pyrogenic silica, which used to control the flow properties of the sealant.
Once fully cured, condensation systems are effective as sealants and caulks in plumbing and building construction and as molds for casting polyurethane, epoxy and polyester resins, waxes, gypsum, and low-melting-temperature metals such as lead. They are typically very flexible and have a high tear strength. They do not require the use of a release agent since silicones have non-stick properties.

 Polysiloxanes differ from other polymers in that their backbones consist of Si–O–Si units unlike many other polymers that contain carbon backbones. Polysiloxane is very flexible due to large bond angles and bond lengths when compared to those found in more basic polymers such as
Polysiloxanes differ from other polymers in that their backbones consist of Si–O–Si units unlike many other polymers that contain carbon backbones. Polysiloxane is very flexible due to large bond angles and bond lengths when compared to those found in more basic polymers such as  The siloxane backbone is a more flexible polymer than the basic carbon chain backbone because the side groups are spaced farther apart. Polymer segments can move farther and change conformation easily, making for a flexible material. Polysiloxanes tend to be more stable and less chemically active because more energy is required to break the silicon-oxygen bond. Although silicon is a congener of carbon, having the same electron bonding configuration, silicon analogues of carbonaceous compounds generally exhibit different properties. The difference in total charge and mass between carbon with 6 protons and 6 neutrons, and silicon with 14 protons and 14 neutrons causes an added layer of electrons and their screening effect changes the electronegativity between the two elements. For example the silicon-oxygen bond in polysiloxanes is significantly more stable than the carbon-oxygen bond in polyoxymethylene, a structurally similar polymer. The difference is partly due to the higher bond energy, the energy required to break the Si-O bond, and also because polyoxymethylene decomposes formaldehyde, which is volatile and escapes driving decomposition forward, but Si-containing decomposition products of silicone are less volatile.
:
The siloxane backbone is a more flexible polymer than the basic carbon chain backbone because the side groups are spaced farther apart. Polymer segments can move farther and change conformation easily, making for a flexible material. Polysiloxanes tend to be more stable and less chemically active because more energy is required to break the silicon-oxygen bond. Although silicon is a congener of carbon, having the same electron bonding configuration, silicon analogues of carbonaceous compounds generally exhibit different properties. The difference in total charge and mass between carbon with 6 protons and 6 neutrons, and silicon with 14 protons and 14 neutrons causes an added layer of electrons and their screening effect changes the electronegativity between the two elements. For example the silicon-oxygen bond in polysiloxanes is significantly more stable than the carbon-oxygen bond in polyoxymethylene, a structurally similar polymer. The difference is partly due to the higher bond energy, the energy required to break the Si-O bond, and also because polyoxymethylene decomposes formaldehyde, which is volatile and escapes driving decomposition forward, but Si-containing decomposition products of silicone are less volatile.
:
 Silicone rubber can be 3d printed (liquid deposition modelling LDM) using pump-nozzle extrusion systems. Unfortunately, standard silicone formulations are optimized to be used by extrusion and injection moulding machines and are not applicable in LDM-based 3D printing. The rheological behavior and the pot life need to be adjusted.
3D printing also requires the use of a removable support material that is compatible with the silicone rubber.
Liquid silicone rubber is also manufactured for
Silicone rubber can be 3d printed (liquid deposition modelling LDM) using pump-nozzle extrusion systems. Unfortunately, standard silicone formulations are optimized to be used by extrusion and injection moulding machines and are not applicable in LDM-based 3D printing. The rheological behavior and the pot life need to be adjusted.
3D printing also requires the use of a removable support material that is compatible with the silicone rubber.
Liquid silicone rubber is also manufactured for
silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cookin ...
—itself a polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
—containing silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
together with carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
, and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost.
Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.
Curing
In its uncured state, silicone rubber is a highly adhesive gel or liquid. To convert it to a solid, it must be cured, vulcanized, or catalyzed. This is normally carried out in a two-stage process at the point of manufacture into the desired shape, and then in a prolonged post-cure process. It can also beinjection molded
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
or 3D printed.
Silicone rubber may be cured by a platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
-catalyzed cure system, a condensation cure system, a peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
cure system, or an oxime cure system. For the platinum-catalyzed cure system, the curing process can be accelerated by adding heat or pressure.
Platinum-based cure system
In a platinum-based silicone cure system, also called an ''addition'' system (because the key reaction-building polymer is an addition reaction), ahydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
- and a vinyl-functional siloxane polymer react in the presence of a platinum complex catalyst, creating an ethyl bridge between the two. The reaction has no byproducts. Such silicone rubbers cure quickly, though the rate of or even ability to cure is easily inhibited in the presence of elemental tin, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, and many amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
compounds.
Condensation cure system
''Condensation curing'' systems can be ''one-part'' or ''two-part'' systems. In one-part or ''RTV'' (room-temperature vulcanizing) system, a cross-linker exposed to ambient humidity (i.e., water) experiences ahydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
step and is left with a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
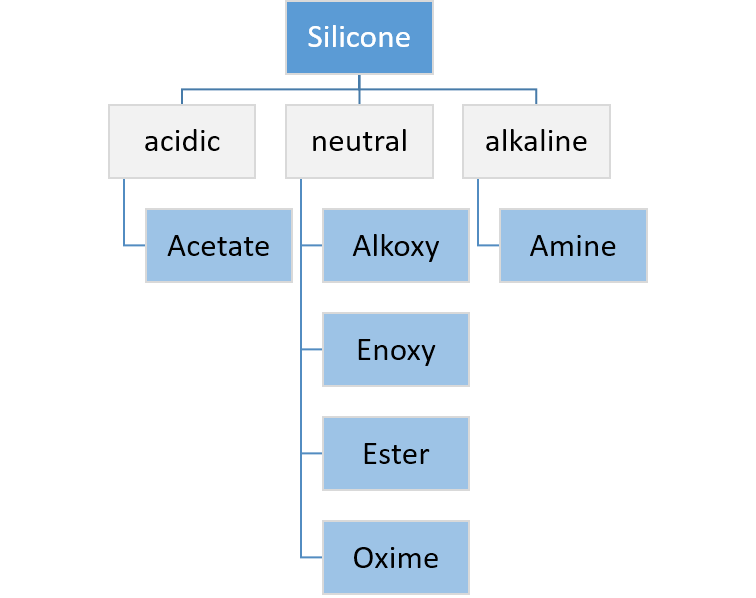
or silanol group. The silanol condenses further with another hydrolyzable group on the polymer or cross-linker and continues until the system is fully cured. Such a system will cure on its own at room temperature and (unlike the platinum-based addition cure system) is not easily inhibited by contact with other chemicals, though the process may be affected by contact with some plastics or metals and may not take place at all if placed in contact with already-cured silicone compounds. The crosslinkers used in condensation cure systems are typically alkoxy, acetoxy, ester, enoxy or oxime silanes such as methyl trimethoxy silane for alkoxy-curing systems and methyl triacetoxysilane for acetoxy-curing systems. In many cases an additional condensation catalyst is added to fully cure the RTV system and achieve a tack-free surface. Organotitanate catalysts such as tetraalkoxy titanates or chelated titanates are used in alkoxy-cured systems. Tin catalysts such as dibutyl tin dilaurate (DBTDL) can be used in oxime and acetoxy-cured systems. Acetoxy tin condensation is one of the oldest cure chemistries used for curing silicone rubber, and is the one used in household bathroom caulk. Depending on the type of detached molecule, it is possible to classify silicone systems as acidic, neutral or alkaline.
Peroxide cure system
Peroxide curing is widely used for curing silicone rubber. The curing process leaves behind byproducts, which can be an issue in food contact and medical applications. However, these products are usually treated in a postcure oven which greatly reduces the peroxide breakdown product content. One of the two mainorganic peroxide
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily b ...
s used, dicumyl peroxide (compare cumene hydroperoxide), has principal breakdown products of acetophenone and phenyl-2-propanol. The other is dichlorobenzoyl peroxide, whose principal breakdown products are dichlorobenzoic acid and dichlorobenzene.

History
The first silicone elastomers were developed in the search for better insulating materials for electric motors and generators. Resin-impregnated glass fibers were the state-of-the-art materials at the time. The glass was very heat resistant, but the phenolic resins would not withstand the higher temperatures that were being encountered in new smaller electric motors. Chemists atCorning Glass
Corning Incorporated is an American multinational technology company that specializes in specialty glass, ceramics, and related materials and technologies including advanced optics, primarily for industrial and scientific applications. The c ...
and General Electric
General Electric Company (GE) is an American multinational conglomerate founded in 1892, and incorporated in New York state and headquartered in Boston. The company operated in sectors including healthcare, aviation, power, renewable ene ...
were investigating heat-resistant materials for use as resinous binders when they synthesized the first silicone polymers, demonstrated that they worked well and found a route to produce them commercially.
The term "silicone" is actually a misnomer. The suffix ''-one'' is used by chemists to denote a substance with a double-bonded atom of oxygen in its backbone. When first discovered, silicone was erroneously believed to have oxygen atoms bonded in this way. Technically correct term for the various silicone rubbers is polysiloxanes ( polydimethylsiloxanes being a large subset), referring to a saturated Si-O backbone.
Corning Glass in a joint venture with Dow Chemical formed Dow Corning in 1943 to produce this new class of materials. As the unique properties of the new silicone products were studied in more detail, their potential for broader usage was envisioned, and GE opened its own plant to produce silicones in 1947. GE Silicones was sold to Momentive Performance Materials in 2006. Wacker Chemie also started production of silicones in Europe in 1947. The Japanese company Shin-Etsu Chemical began mass production of silicone in 1953.
Properties
Silicone rubber offers good resistance to extreme temperatures, being able to operate normally from . Silicone rubber has low tensile strength, poor wear and tear wear properties. Some properties such as elongation,creep
Creep, Creeps or CREEP may refer to:
People
* Creep, a creepy person
Politics
* Committee for the Re-Election of the President (CRP), mockingly abbreviated as CREEP, an fundraising organization for Richard Nixon's 1972 re-election campaign
Art ...
, cyclic flexing, tear strength
Tear resistance (or tear strength) is a measure of how well a material can withstand the effects of tearing. It is a useful engineering measurement for a wide variety of materials by many different test methods.
Discussion
For example, with ru ...
, compression set, dielectric strength (at high voltage), thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
, fire resistance and in some cases tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials ...
can be—at extreme temperatures—far superior to organic rubbers in general, although a few of these properties are still lower than for some specialty materials. Silicone rubber is a material of choice in industry when retention of initial shape and mechanical strength are desired under heavy thermal stress or sub-zero temperatures.
Compared to organic rubber
Organic rubber has a carbon-to-carbon backbone which can leave it susceptible toozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the l ...
, UV, heat and other aging factors that silicone rubber can withstand well. This makes silicone rubber one of the elastomers of choice in many extreme environments. Silicone is considerably more permeable to gasses than most other rubbers which limits its use in some areas.
Silicone rubber is highly inert and does not react with most chemicals and isn’t available to participate in biological processes allowing it to be used in many medical applications including medical implant
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, whi ...
s.
It is biocompatible, hypoallergenic
Hypoallergenic, meaning "below average" or "slightly" allergenic, is a term meaning that something (usually cosmetics, pets, textiles, food, etc.) causes fewer allergic reactions. The term was first used in 1953 in an advertising campaign for cos ...
, which makes it suitable for baby care products, and food contact in general.
Silicone rubber is a reliable solution (as opposed to rubber and thermoplastic elastomers) for migration or interaction problems between the main active ingredients. Its chemical stability prevents it from affecting any substrate it is in contact with (skin, water, blood, active ingredients, etc.).
:
Production
To make silicone, the silicon atoms must be isolated from the silicon dioxide compoundsilica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
. This is done by heating large volumes of quartz sand to extremely high temperatures, often up to 1800 °C. From here, there are several processes where silicon is combined with methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industria ...
and heated. It is then distilled into a polymerised siloxane known as polydimethylsiloxane. The polydimethylsiloxane can then be polymerised. This is done using a variety of techniques depending on the use of the final product. The raw silicone compound is combined with any desired additives, which may include pigments, and the catalyst. It is then injection moulded
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
, extruded or 3D printed. Curing is the final stage in the production process.
Structure
polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including b ...
. For example, a C–C backbone unit has a bond length of 1.54 Å and a bond angle of 112°, whereas the siloxane backbone unit Si–O has a bond length of 1.63 Å and a bond angle of 130°.  The siloxane backbone is a more flexible polymer than the basic carbon chain backbone because the side groups are spaced farther apart. Polymer segments can move farther and change conformation easily, making for a flexible material. Polysiloxanes tend to be more stable and less chemically active because more energy is required to break the silicon-oxygen bond. Although silicon is a congener of carbon, having the same electron bonding configuration, silicon analogues of carbonaceous compounds generally exhibit different properties. The difference in total charge and mass between carbon with 6 protons and 6 neutrons, and silicon with 14 protons and 14 neutrons causes an added layer of electrons and their screening effect changes the electronegativity between the two elements. For example the silicon-oxygen bond in polysiloxanes is significantly more stable than the carbon-oxygen bond in polyoxymethylene, a structurally similar polymer. The difference is partly due to the higher bond energy, the energy required to break the Si-O bond, and also because polyoxymethylene decomposes formaldehyde, which is volatile and escapes driving decomposition forward, but Si-containing decomposition products of silicone are less volatile.
:
The siloxane backbone is a more flexible polymer than the basic carbon chain backbone because the side groups are spaced farther apart. Polymer segments can move farther and change conformation easily, making for a flexible material. Polysiloxanes tend to be more stable and less chemically active because more energy is required to break the silicon-oxygen bond. Although silicon is a congener of carbon, having the same electron bonding configuration, silicon analogues of carbonaceous compounds generally exhibit different properties. The difference in total charge and mass between carbon with 6 protons and 6 neutrons, and silicon with 14 protons and 14 neutrons causes an added layer of electrons and their screening effect changes the electronegativity between the two elements. For example the silicon-oxygen bond in polysiloxanes is significantly more stable than the carbon-oxygen bond in polyoxymethylene, a structurally similar polymer. The difference is partly due to the higher bond energy, the energy required to break the Si-O bond, and also because polyoxymethylene decomposes formaldehyde, which is volatile and escapes driving decomposition forward, but Si-containing decomposition products of silicone are less volatile.
:
Special grades
There are many special grades and forms of silicone rubber, including:steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporizatio ...
resistant, metal detectable, high tear strength, extreme high temperature, extreme low temperature, electrically conductive, chemical/oil/acid/gas resistant, low smoke emitting, and flame-retardant. A variety of fillers can be used in silicone rubber, although most are non-reinforcing and lower the tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials ...
.
Silicone rubber is available in a range of hardness levels, expressed as Shore A or IRHD between 10 and 100, the higher number being the harder compound. It is also available in virtually any colour, and can be colour matched.
Applications
Silicone rubber is used in automotive applications, many cooking, baking, and food storage products, apparel including undergarments, sportswear, and footwear, electronics, to home repair and hardware, and a host of unseen applications. It is usually processed and shaped with the following methods.Extrusion
Once mixed and coloured, silicone rubber can be extruded into tubes, strips, solid cord or custom profiles according to the size specifications of the manufacturer. Cord can be joined to make O-rings and extruded profiles can be joined to make seals.Injection moulding
Silicone rubber can be moulded into custom shapes and designs. Manufacturers work to set industry tolerances when extruding, cutting or joining silicone rubber profiles. In the UK this is BS 3734, for extrusions the tightest level is E1 and the widest is E3.3D printing
life science
Life is a quality that distinguishes matter that has biological processes, such as signaling and self-sustaining processes, from that which does not, and is defined by the capacity for growth, reaction to stimuli, metabolism, energy ...
applications (syringe pistons, closure for dispensing system, gaskets for IV flow regulator, respiratory masks, implantable chambers for IV administration), cosmetic products (Mascara brush, make-up packaging, make-up applicator and lipstick moulds) and optics products (circular lens, collimators, Fresnel lenses and free form lenses).Freeze-tolerant solar water-heating panels exploit the elasticity of silicone to repeatedly accommodate the expansion of water on freezing, while its extreme temperature tolerance maintain a lack of brittleness below freezing and excellent tolerance of temperatures in excess of . Its property of not having a carbon backbone, but a chemically robust silicon backbone instead, reduces its potential as a food source for dangerous waterborne bacteria such as Legionella.
Non-dyed silicone rubber tape with an iron(III) oxide additive (making the tape a red-orange colour) is used extensively in aviation and aerospace wiring applications as a splice or wrapping tape due to its non-flammable nature. The iron oxide additive adds high thermal conductivity but does not change the high electrical insulation property of the silicone rubber. This type of self-amalgamating tape amalgamates or fuses to itself, so that when stretched and wrapped around cables, electrical joints, hoses and pipes it bonds into a strong seamless rubbery electrically insulating and waterproof layer, although not adhesive.
As an electrical insulator, silicone rubber has the added virtue of remaining non-conductive when damaged by heat, reducing the likelihood of runaway arcing.
With the addition of carbon or another conductive substance as a powdered filler, silicone rubber can be made electrically conductive while retaining most of its other mechanical properties. As such it is used for flexible contacts which close on being pressed, used in many devices such as computer keyboard
A computer keyboard is a peripheral input device modeled after the typewriter keyboard which uses an arrangement of buttons or keys to act as mechanical levers or electronic switches. Replacing early punched cards and paper tape technolog ...
s and remote control
In electronics, a remote control (also known as a remote or clicker) is an electronic device used to operate another device from a distance, usually wirelessly. In consumer electronics, a remote control can be used to operate devices such ...
handsets.
Self-healing
In 2007, silicone rubber formed the matrix of the first autonomic self-healing elastomer.Keller ''et al.'', ''A Self-Healing Poly(dimethyl siloxane) Elastomer,'' Advanced Functional Materials, v. 17, p. 2399–2404 (2007). The microcapsule-based material was capable of recovering almost all of the original tear strength. Additionally, this material had improved fatigue properties as evaluated using a torsion-fatigue test.Keller ''et al.'', ''Torsion Fatigue Response of Self-Healing Poly(dimethyl siloxane) Elastomers'', Polymer, v.49 p. 3136–3145 (2008).See also
*Injection molding of liquid silicone rubber
Injection molding of liquid silicone rubber (LSR) is a process to produce pliable, durable parts in high volume.
Liquid silicone rubber is a high purity platinum cured silicone with low compression set, good stability and ability to resist ext ...
* Forensic engineering
* Forensic polymer engineering
* Medical grade silicone
* RTV silicone
References
Further reading
* Brydson, John (1999) ''Plastics Materials'', Butterworth, 9th Ed * Lewis, PR, Reynolds, K and Gagg, C (2004) ''Forensic Materials Engineering: Case Studies'', CRC Press {{rubber Elastomers Sculpture materials * * de:Silikone#Silikonkautschuk zh:硅橡胶