SN1 reaction on:
[Wikipedia]
[Google]
[Amazon]
The SN1 reaction is a
 This SN1 reaction takes place in three steps:
* Formation of a ''tert''-butyl carbocation by separation of a
This SN1 reaction takes place in three steps:
* Formation of a ''tert''-butyl carbocation by separation of a  :
: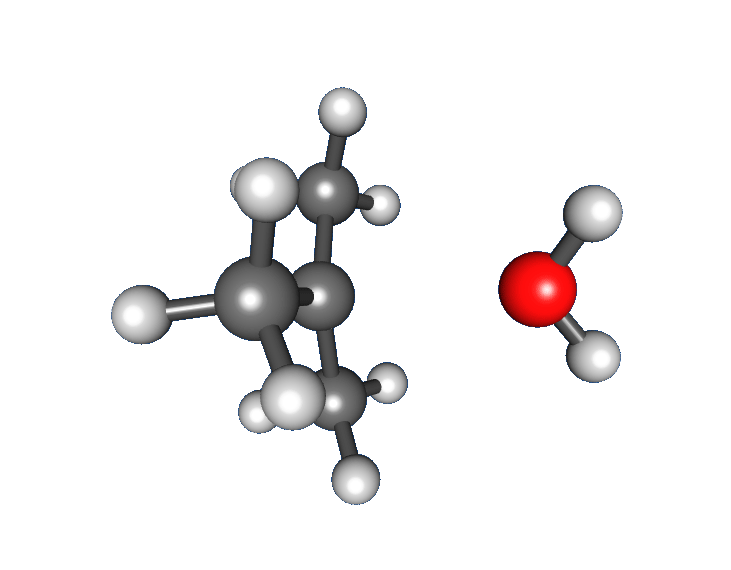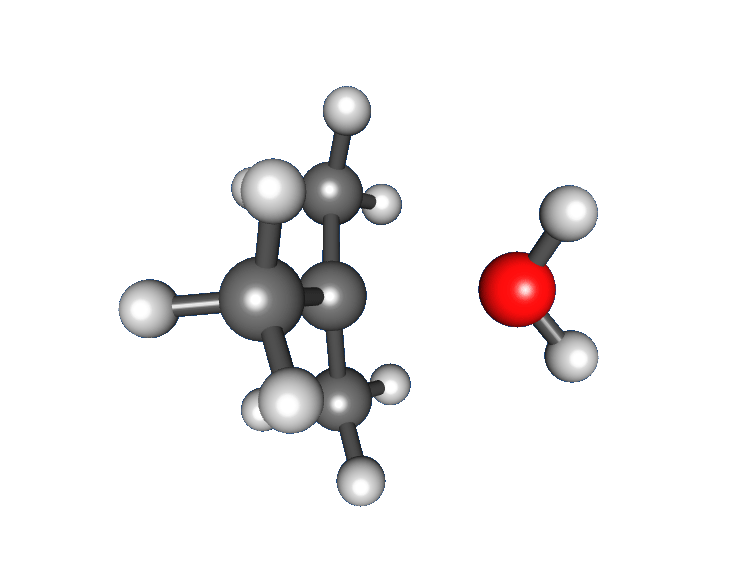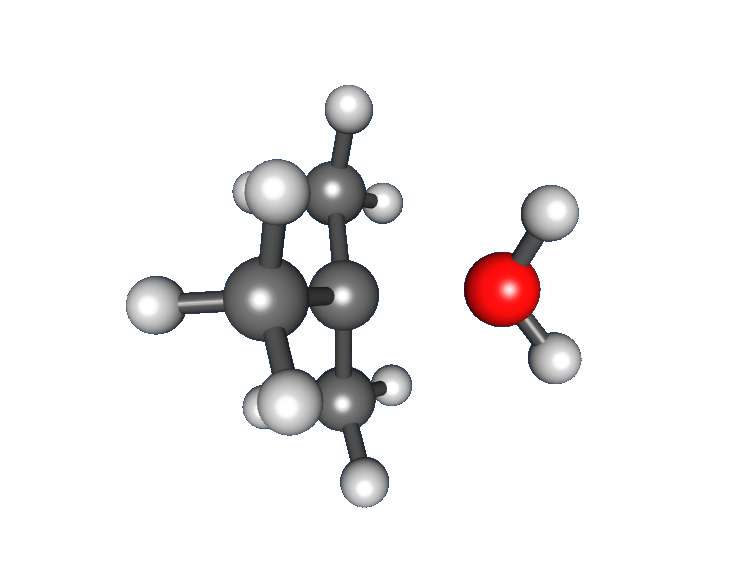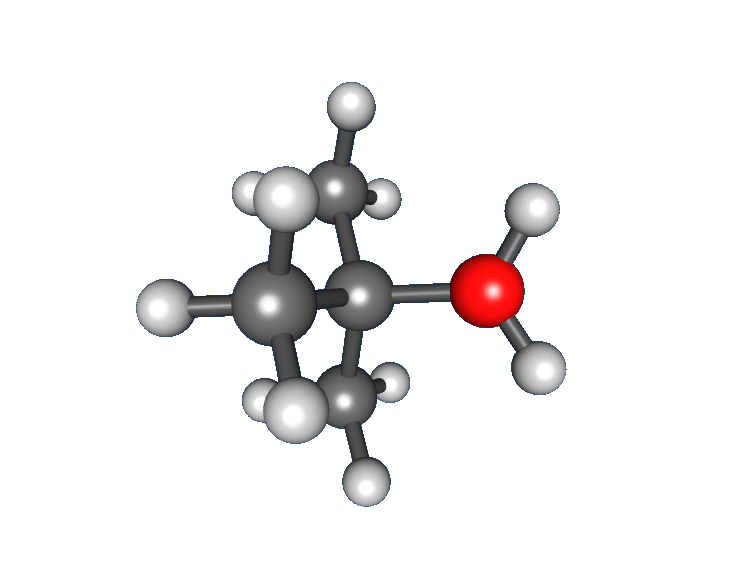 * Nucleophilic attack: the carbocation reacts with the nucleophile. If the
* Nucleophilic attack: the carbocation reacts with the nucleophile. If the  *
* 
 Though a relatively stable tertiary
Though a relatively stable tertiary
 As the alpha and beta substitutions increase with respect to leaving groups, the reaction is diverted from SN2 to SN1.
As the alpha and beta substitutions increase with respect to leaving groups, the reaction is diverted from SN2 to SN1.
 However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted as the nucleophile comes in from the rear side of the leaving group.
However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted as the nucleophile comes in from the rear side of the leaving group.
Diagrams
Exercise
the University of Maine {{DEFAULTSORT:Sn1 Reaction Nucleophilic substitution reactions Reaction mechanisms
substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
", and the "1" says that the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
is unimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coeffic ...
. Thus, the rate equation
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reac ...
is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
intermediate and is commonly seen in reactions of secondary or tertiary alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
s under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction occurs. In inorganic chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disci ...
, the SN1 reaction is often known as the ''dissociative substitution
In chemistry, dissociative substitution describes a Chemical reaction, reaction pathway by which Chemical compound, compounds interchange ligands. The term is typically applied to Coordination chemistry, coordination and Organometallic chemistry ...
''. This dissociation pathway is well-described by the cis effect. A reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
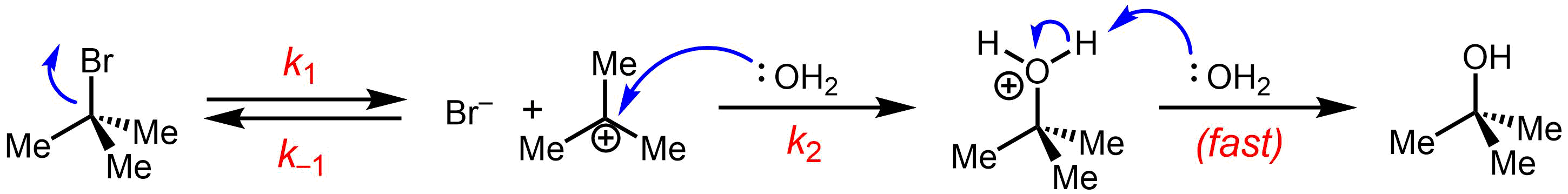
was first proposed by Christopher Ingold et al. in 1940. This reaction does not depend much on the strength of the nucleophile, unlike the SN2 mechanism. This type of mechanism involves two steps. The first step is the ionization of alkyl halide in the presence of aqueous acetone or ethyl alcohol. This step provides a carbocation as an intermediate.
In the first step of SN1 mechanism, a carbocation is formed which is planar and hence attack of nucleophile (second step) may occur from either side to give a racemic product, but actually complete racemization does not take place. This is because the nucleophilic species attacks the carbocation even before the departing halides ion has moved sufficiently away from the carbocation. The negatively charged halide ion shields the carbocation from being attacked on the front side, and backside attack, which leads to inversion of configuration, is preferred. Thus the actual product no doubt consists of a mixture of enantiomers but the enantiomers with inverted configuration would predominate and complete racemization does not occurs.
Mechanism
An example of a reaction taking place with an SN1reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
is the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
of tert-butyl bromide forming ''tert''-butanol:
:leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
(a bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant ...
anion) from the carbon atom: this step is slow.
: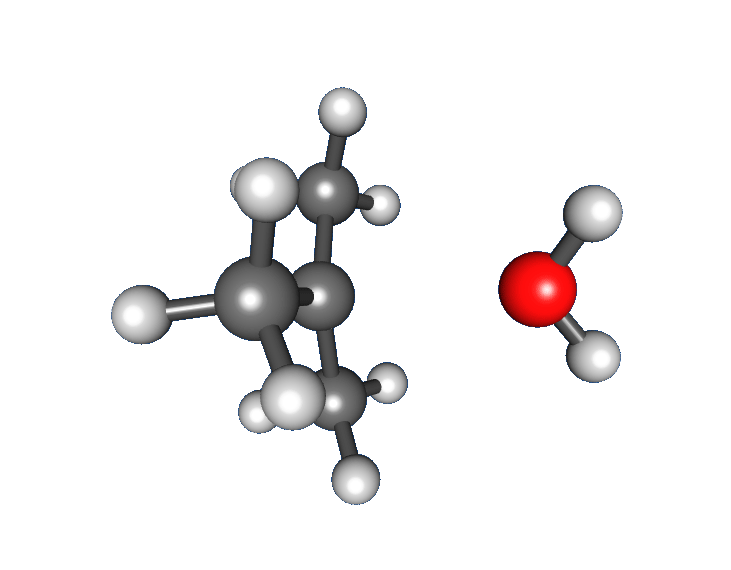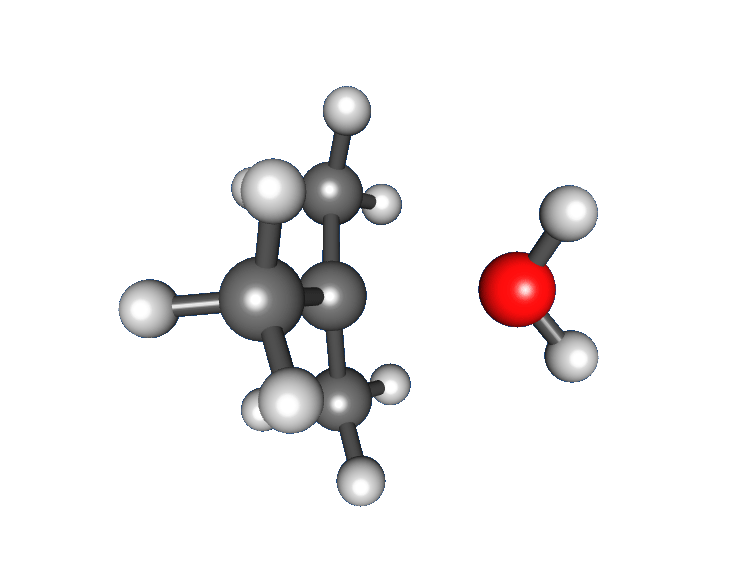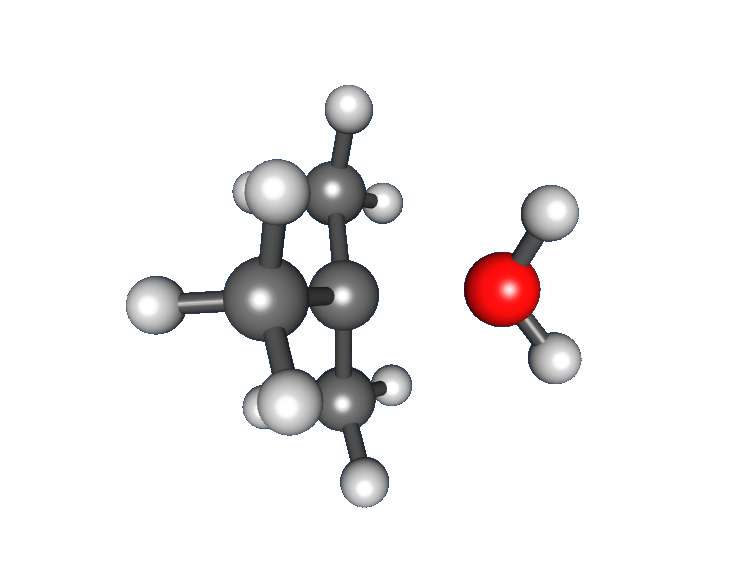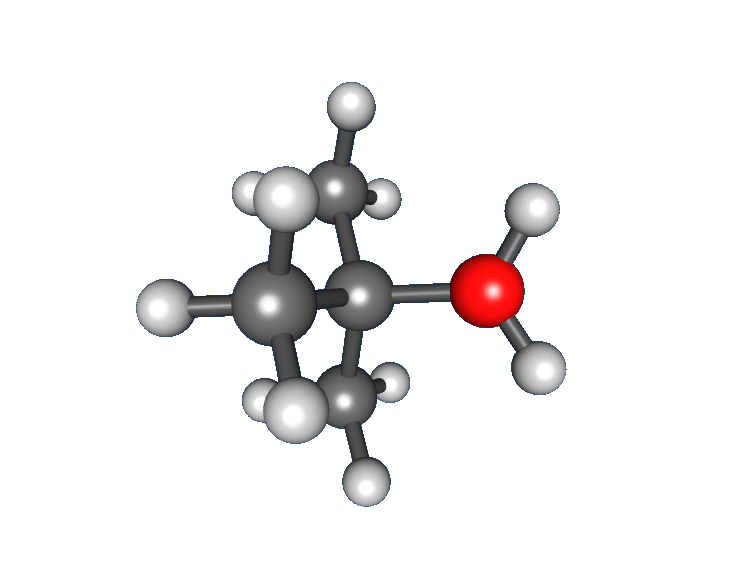 * Nucleophilic attack: the carbocation reacts with the nucleophile. If the
* Nucleophilic attack: the carbocation reacts with the nucleophile. If the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
is a neutral molecule (i.e. a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
) a third step is required to complete the reaction. When the solvent is water, the intermediate is an oxonium ion. This reaction step is fast.
:Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
: Removal of a proton on the protonated nucleophile by water acting as a base forming the alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
and a hydronium ion. This reaction step is fast.
Rate law
Although the rate law of the SN1 reaction is often regarded as being first order in alkyl halide and zero order in nucleophile, this is a simplification that holds true only under certain conditions. While it, too, is an approximation, the rate law derived from the steady state approximation (SSA) provides more insight into the kinetic behavior of the SN1 reaction. Consider the following reaction scheme for the mechanism shown above: Though a relatively stable tertiary
Though a relatively stable tertiary carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
, ''tert''-butyl cation is a high-energy species that is present only at very low concentration and cannot be directly observed under normal conditions. Thus, the SSA can be applied to this species:
* (1) Steady state assumption: ''d'' 't''Bu+''dt'' = 0 = ''k''1 't''BuBr– ''k–''1 't''Bu+Br–] – ''k''2 't''Bu+H2O]
* (2) Concentration of ''t''-butyl cation, based on steady state assumption: 't''Bu+= ''k''1 't''BuBr(''k–''1 r–+ ''k''2 2O
* (3) Overall reaction rate, assuming rapid final step: ''d'' 't''BuOH''dt'' = ''k''2 't''Bu+H2O]
* (4) Steady state rate law, by plugging (2) into (3): ''d'' 't''BuOH''dt'' = ''k''1''k''2 't''BuBrH2O]/(''k–''1 r–+ ''k''2 2O
Under normal synthetic conditions, the entering nucleophile is more nucleophilic than the leaving group and is present in excess. Moreover, kinetic experiments are often conducted under initial rate conditions (5 to 10% conversion) and without the addition of bromide, so r–is negligible. For these reasons, ''k–''1 r–≪ ''k''2 2Ooften holds. Under these conditions, the SSA rate law reduces to
rate = ''d'' 't''BuOH''dt'' = ''k''1''k''2 't''BuBrH2O]/(''k''2 2O = ''k''1 't''BuBr
the simple first-order rate law described in introductory textbooks. Under these conditions, the concentration of the nucleophile does not affect the rate of the reaction, and changing the nucleophile (e.g. from H2O to MeOH) does not affect the reaction rate, though the product is, of course, different. In this regime, the first step (ionization of the alkyl bromide) is slow, rate-determining, and irreversible, while the second step (nucleophilic addition) is fast and kinetically invisible.
However, under certain conditions, non-first-order reaction kinetics can be observed. In particular, when a large concentration of bromide is present while the concentration of water is limited, the reverse of the first step becomes important kinetically. As the SSA rate law indicates, under these conditions there is a fractional (between zeroth and first order) dependence on 2O while there is a negative fractional order dependence on r– Thus, SN1 reactions are often observed to slow down when an exogenous source of the leaving group (in this case, bromide) is added to the reaction mixture. This is known as the '' common ion effect'' and the observation of this effect is evidence for an SN1 mechanism (although the absence of a common ion effect does not rule it out).
Scope
The SN1 mechanism tends to dominate when the central carbon atom is surrounded by bulky groups because such groups sterically hinder the SN2 reaction. Additionally, bulky substituents on the central carbon increase the rate of carbocation formation because of the relief of steric strain that occurs. The resultant carbocation is also stabilized by both inductive stabilization and hyperconjugation from attachedalkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
groups. The Hammond–Leffler postulate suggests that this, too, will increase the rate of carbocation formation. The SN1 mechanism therefore dominates in reactions at tertiary alkyl centers.
An example of a reaction proceeding in a SN1 fashion is the synthesis of ''2,5-dichloro-2,5-dimethylhexane'' from the corresponding diol with concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
:
:Stereochemistry
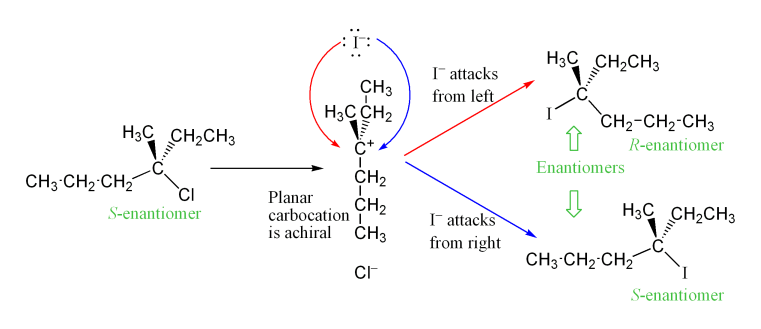
The carbocation intermediate formed in the reaction's rate determining step (RDS) is an ''sp2'' hybridized carbon with trigonal planar molecular geometry. This allows two different ways for the nucleophilic attack, one on either side of the planar molecule. If neither approach is favored, then these two ways occur equally, yielding aracemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
of enantiomers if the reaction takes place at a stereocenter. This is illustrated below in the SN1 reaction of S-3-chloro-3-methylhexane with an iodide ion, which yields a racemic mixture of 3-iodo-3-methylhexane:
 However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted as the nucleophile comes in from the rear side of the leaving group.
However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted as the nucleophile comes in from the rear side of the leaving group.
Side reactions
Two common side reactions areelimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
s and carbocation rearrangement. If the reaction is performed under warm or hot conditions (which favor an increase in entropy), E1 elimination is likely to predominate, leading to formation of an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
. At lower temperatures, SN1 and E1 reactions are competitive reactions and it becomes difficult to favor one over the other. Even if the reaction is performed cold, some alkene may be formed. If an attempt is made to perform an SN1 reaction using a strongly basic nucleophile such as hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
or methoxide ion, the alkene will again be formed, this time via an E2 elimination
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
. This will be especially true if the reaction is heated. Finally, if the carbocation intermediate can rearrange to a more stable carbocation, it will give a product derived from the more stable carbocation rather than the simple substitution product.
Solvent effects
Since the SN1 reaction involves formation of an unstable carbocation intermediate in the rate-determining step (RDS), anything that can facilitate this process will speed up the reaction. The normal solvents of choice are both ''polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
*Polar climate, the cli ...
'' (to stabilize ionic intermediates in general) and ''protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile ...
s'' (to solvate
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the ...
the leaving group in particular). Typical polar protic solvents include water and alcohols, which will also act as nucleophiles, and the process is known as solvolysis.
The Y scale correlates solvolysis reaction rates of any solvent (k) with that of a standard solvent (80% v/v ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
/water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
) (k0) through
:
with m a reactant constant (m = 1 for ''tert''-butyl chloride) and Y a solvent parameter. For example, 100% ethanol gives Y = −2.3, 50% ethanol in water Y = +1.65 and 15% concentration Y = +3.2.
See also
* Arrow pushing * Nucleophilic acyl substitution * Neighbouring group participation * SN2 reactionReferences
External links
Diagrams
Frostburg State University
Frostburg State University (FSU) is a public university in Frostburg, Maryland. The university is the only four-year institution of the University System of Maryland west of the Baltimore-Washington passageway in the state's Appalachian highlan ...
Exercise
the University of Maine {{DEFAULTSORT:Sn1 Reaction Nucleophilic substitution reactions Reaction mechanisms