Reptiles, as most commonly defined are the animals in the
class Reptilia ( ), a
paraphyletic grouping comprising all
sauropsids except
birds.
Living reptiles comprise
turtle
Turtles are an order of reptiles known as Testudines, characterized by a special shell developed mainly from their ribs. Modern turtles are divided into two major groups, the Pleurodira (side necked turtles) and Cryptodira (hidden necked t ...
s,
crocodilia
Crocodilia (or Crocodylia, both ) is an order of mostly large, predatory, semiaquatic reptiles, known as crocodilians. They first appeared 95 million years ago in the Late Cretaceous period ( Cenomanian stage) and are the closest livi ...
ns,
squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
(
lizards and
snake
Snakes are elongated, limbless, carnivorous reptiles of the suborder Serpentes . Like all other squamates, snakes are ectothermic, amniote vertebrates covered in overlapping scales. Many species of snakes have skulls with several more j ...
s) and
rhynchocephalians (
tuatara
Tuatara (''Sphenodon punctatus'') are reptiles endemic to New Zealand. Despite their close resemblance to lizards, they are part of a distinct lineage, the order Rhynchocephalia. The name ''tuatara'' is derived from the Māori language and m ...
). As of March 2022, the
Reptile Database
The Reptile Database is a scientific database that collects taxonomic information on all living reptile species (i.e. no fossil species such as dinosaurs). The database focuses on species (as opposed to higher ranks such as families) and has entrie ...
includes about 11,700 species. In the traditional
Linnaean classification system, birds are considered a separate class to reptiles. However, crocodilians are more closely related to birds than they are to other living reptiles, and so modern
cladistic
Cladistics (; ) is an approach to biological classification in which organisms are categorized in groups ("clades") based on hypotheses of most recent common ancestry. The evidence for hypothesized relationships is typically shared derived char ...
classification systems include birds within Reptilia, redefining the term as a
clade. Other cladistic definitions abandon the term reptile altogether in favor of the clade
Sauropsida, which refers to all amniotes more closely related to modern reptiles than to mammals. The study of the traditional reptile
orders, historically combined with that of modern
amphibians, is called
herpetology.
The earliest known proto-reptiles originated around 312 million years ago during the
Carboniferous period, having evolved from advanced
reptiliomorph
Reptiliomorpha (meaning reptile-shaped; in PhyloCode known as ''Pan-Amniota'') is a clade containing the amniotes and those tetrapods that share a more recent common ancestor with amniotes than with living amphibians (lissamphibians). It was defi ...
tetrapods which became increasingly adapted to life on dry land. The earliest known
eureptile
Eureptilia ("true reptiles") is one of the two major subgroups of the clade Sauropsida, the other one being Parareptilia. Eureptilia includes Diapsida (the clade containing all modern reptiles and birds), as well as a number of primitive Permo-C ...
("true reptile") was ''
Hylonomus
''Hylonomus'' (; ''hylo-'' "forest" + ''nomos'' "dweller") is an extinct genus of reptile that lived 312 million years ago during the Late Carboniferous period.
It is the earliest unquestionable reptile (''Westlothiana'' is older, but in fact it ...
,'' a small and superficially lizard-like animal. Genetic and fossil data argues that the two largest lineages of reptiles,
Archosauromorpha (crocodilians, birds, and kin) and
Lepidosauromorpha (lizards, and kin), diverged near the end of the
Permian
The Permian ( ) is a geologic period and System (stratigraphy), stratigraphic system which spans 47 million years from the end of the Carboniferous Period million years ago (Mya), to the beginning of the Triassic Period 251.9 Mya. It is the last ...
period.
In addition to the living reptiles, there are many diverse groups that are now
extinct, in some cases due to
mass extinction events. In particular, the
Cretaceous–Paleogene extinction event
The Cretaceous–Paleogene (K–Pg) extinction event (also known as the Cretaceous–Tertiary extinction) was a sudden mass extinction of three-quarters of the plant and animal species on Earth, approximately 66 million years ago. With the ...
wiped out the
pterosaur
Pterosaurs (; from Greek ''pteron'' and ''sauros'', meaning "wing lizard") is an extinct clade of flying reptiles in the order, Pterosauria. They existed during most of the Mesozoic: from the Late Triassic to the end of the Cretaceous (228 ...
s,
plesiosaurs
The Plesiosauria (; Greek: πλησίος, ''plesios'', meaning "near to" and ''sauros'', meaning "lizard") or plesiosaurs are an order or clade of extinct Mesozoic marine reptiles, belonging to the Sauropterygia.
Plesiosaurs first appeared i ...
, and all non-avian
dinosaurs alongside many species of
crocodyliforms
Crocodyliformes is a clade of crurotarsan archosaurs, the group often traditionally referred to as "crocodilians". They are the first members of Crocodylomorpha to possess many of the features that define later relatives. They are the only pseudo ...
, and
squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
(e.g.,
mosasaur
Mosasaurs (from Latin ''Mosa'' meaning the 'Meuse', and Greek ' meaning 'lizard') comprise a group of extinct, large marine reptiles from the Late Cretaceous. Their first fossil remains were discovered in a limestone quarry at Maastricht on ...
s). Modern non-bird reptiles inhabit all the continents except
Antarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean, it contains the geographic South Pole. Antarctica is the fifth-largest cont ...
.
Reptiles are tetrapod
vertebrates
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () (chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, with c ...
, creatures that either have four limbs or, like snakes, are descended from four-limbed ancestors. Unlike
amphibians, reptiles do not have an aquatic larval stage. Most reptiles are
oviparous, although several species of squamates are
viviparous
Among animals, viviparity is development of the embryo inside the body of the parent. This is opposed to oviparity which is a reproductive mode in which females lay developing eggs that complete their development and hatch externally from the ...
, as were some extinct aquatic clades
– the fetus develops within the mother, using a
(non-mammalian) placenta rather than contained in an
eggshell. As amniotes, reptile eggs are surrounded by membranes for protection and transport, which adapt them to reproduction on dry land. Many of the viviparous species feed their
fetus
A fetus or foetus (; plural fetuses, feti, foetuses, or foeti) is the unborn offspring that develops from an animal embryo. Following embryonic development the fetal stage of development takes place. In human prenatal development, fetal dev ...
es through various forms of placenta analogous to those of
mammals, with some providing initial care for their hatchlings.
Extant reptiles range in size from a tiny gecko, ''
Sphaerodactylus ariasae'', which can grow up to to the
saltwater crocodile
The saltwater crocodile (''Crocodylus porosus'') is a crocodilian native to saltwater habitats and brackish wetlands from India's east coast across Southeast Asia and the Sundaic region to northern Australia and Micronesia. It has been list ...
, ''Crocodylus porosus'', which can reach over in length and weigh over .
Classification
Research history

In the 13th century the category of ''reptile'' was recognized in Europe as consisting of a miscellany of egg-laying creatures, including "snakes, various fantastic monsters, lizards, assorted amphibians, and worms", as recorded by
Vincent of Beauvais
Vincent of Beauvais ( la, Vincentius Bellovacensis or ''Vincentius Burgundus''; c. 1264) was a Dominican friar at the Cistercian monastery of Royaumont Abbey, France. He is known mostly for his ''Speculum Maius'' (''Great mirror''), a major work ...
in his ''Mirror of Nature''.
In the 18th century, the reptiles were, from the outset of classification, grouped with the
amphibians.
Linnaeus
Carl Linnaeus (; 23 May 1707 – 10 January 1778), also known after his ennoblement in 1761 as Carl von Linné Blunt (2004), p. 171. (), was a Swedish botanist, zoologist, taxonomist, and physician who formalised binomial nomenclature, the ...
, working from species-poor
Sweden, where the
common adder
''Vipera berus'', the common European adderMallow D, Ludwig D, Nilson G. (2003). ''True Vipers: Natural History and Toxinology of Old World Vipers''. Malabar, Florida: Krieger Publishing Company. . or common European viper,Stidworthy J. (1974). ...
and
grass snake
The grass snake (''Natrix natrix''), sometimes called the ringed snake or water snake, is a Eurasian non-venomous colubrid snake. It is often found near water and feeds almost exclusively on amphibians.
Subspecies
Many subspecies are recognized ...
are often found hunting in water, included all reptiles and amphibians in
class "III –
Amphibia
Amphibians are four-limbed and ectothermic vertebrates of the class Amphibia. All living amphibians belong to the group Lissamphibia. They inhabit a wide variety of habitats, with most species living within terrestrial, fossorial, arbor ...
" in his ''
Systema Naturæ''.
The terms ''reptile'' and ''amphibian'' were largely interchangeable, ''reptile'' (from Latin ''repere'', 'to creep') being preferred by the French.
Josephus Nicolaus Laurenti
Josephus Nicolaus Laurenti (4 December 1735, Vienna – 17 February 1805, Vienna) was an Austrian naturalist and zoologist of Italian origin.
Laurenti is considered the auctor of the class Reptilia ( reptiles) through his authorship of ' (1 ...
was the first to formally use the term ''Reptilia'' for an expanded selection of reptiles and amphibians basically similar to that of Linnaeus. Today, the two groups are still commonly treated under the single heading
herpetology.

It was not until the beginning of the 19th century that it became clear that reptiles and amphibians are, in fact, quite different animals, and
Pierre André Latreille
Pierre André Latreille (; 29 November 1762 – 6 February 1833) was a French zoologist, specialising in arthropods. Having trained as a Roman Catholic priest before the French Revolution, Latreille was imprisoned, and only regained his freedom ...
erected the class ''Batracia'' (1825) for the latter, dividing the
tetrapod
Tetrapods (; ) are four-limbed vertebrate animals constituting the superclass Tetrapoda (). It includes extant and extinct amphibians, sauropsids ( reptiles, including dinosaurs and therefore birds) and synapsids ( pelycosaurs, extinct t ...
s into the four familiar classes of reptiles, amphibians, birds, and mammals. The British anatomist
Thomas Henry Huxley
Thomas Henry Huxley (4 May 1825 – 29 June 1895) was an English biologist and anthropologist specialising in comparative anatomy. He has become known as "Darwin's Bulldog" for his advocacy of Charles Darwin's theory of evolution.
The stori ...
made Latreille's definition popular and, together with
Richard Owen, expanded Reptilia to include the various fossil "
antediluvian
The antediluvian (alternatively pre-diluvian or pre-flood) period is the time period chronicled in the Bible between the fall of man and the Genesis flood narrative in biblical cosmology. The term was coined by Thomas Browne. The narrative takes ...
monsters", including
dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s and the mammal-like (
synapsid
Synapsids + (, 'arch') > () "having a fused arch"; synonymous with ''theropsids'' (Greek, "beast-face") are one of the two major groups of animals that evolved from basal amniotes, the other being the sauropsids, the group that includes reptil ...
) ''
Dicynodon'' he helped describe. This was not the only possible classification scheme: In the Hunterian lectures delivered at the
Royal College of Surgeons
The Royal College of Surgeons is an ancient college (a form of corporation) established in England to regulate the activity of surgeons. Derivative organisations survive in many present and former members of the Commonwealth. These organisations a ...
in 1863, Huxley grouped the vertebrates into
mammals, sauroids, and ichthyoids (the latter containing the fishes and amphibians). He subsequently proposed the names of
Sauropsida and
Ichthyopsida for the latter two groups. In 1866,
Haeckel demonstrated that vertebrates could be divided based on their reproductive strategies, and that reptiles, birds, and mammals were united by the
amniotic egg.
The terms ''Sauropsida'' ('lizard faces') and ''
Theropsida'' ('beast faces') were used again in 1916 by
E.S. Goodrich to distinguish between lizards, birds, and their relatives on the one hand (Sauropsida) and
mammals and their extinct relatives (Theropsida) on the other. Goodrich supported this division by the nature of the hearts and blood vessels in each group, and other features, such as the structure of the forebrain. According to Goodrich, both lineages evolved from an earlier stem group, Protosauria ("first lizards") in which he included some animals today considered
reptile-like amphibians, as well as early reptiles.
In 1956,
D.M.S. Watson observed that the first two groups diverged very early in reptilian history, so he divided Goodrich's Protosauria between them. He also reinterpreted Sauropsida and Theropsida to exclude birds and mammals, respectively. Thus his Sauropsida included
Procolophonia,
Eosuchia
Eosuchians are an extinct order of diapsid reptiles. Depending on which taxa are included the order may have ranged from the late Carboniferous to the Eocene but the consensus is that eosuchians are confined to the Permian and Triassic.
Eosuchi ...
,
Millerosauria
Millerosauria is an order of Parareptiles that contains the families †Millerettidae and †Eunotosauridae. It is the sister group to the order Procolophonomorpha. It was named in 1957 by Watson. It was once considered a suborder of the disused ...
,
Chelonia
The green sea turtle (''Chelonia mydas''), also known as the green turtle, black (sea) turtle or Pacific green turtle, is a species of large sea turtle of the family Cheloniidae. It is the only species in the genus ''Chelonia''. Its range exten ...
(turtles),
Squamata
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species ...
(lizards and snakes),
Rhynchocephalia,
Crocodilia
Crocodilia (or Crocodylia, both ) is an order of mostly large, predatory, semiaquatic reptiles, known as crocodilians. They first appeared 95 million years ago in the Late Cretaceous period ( Cenomanian stage) and are the closest livi ...
, "
thecodonts" (
paraphyletic basal Archosauria), non-
avian dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s,
pterosaur
Pterosaurs (; from Greek ''pteron'' and ''sauros'', meaning "wing lizard") is an extinct clade of flying reptiles in the order, Pterosauria. They existed during most of the Mesozoic: from the Late Triassic to the end of the Cretaceous (228 ...
s,
ichthyosaurs, and
sauropterygia
Sauropterygia ("lizard flippers") is an extinct taxon of diverse, aquatic reptiles that developed from terrestrial ancestors soon after the end-Permian extinction and flourished during the Triassic before all except for the Plesiosauria became ...
ns.
In the late 19th century, a number of definitions of Reptilia were offered. The traits listed by
Lydekker in 1896, for example, include a single
occipital condyle
The occipital condyles are undersurface protuberances of the occipital bone in vertebrates, which function in articulation with the superior facets of the atlas vertebra.
The condyles are oval or reniform (kidney-shaped) in shape, and their anteri ...
, a jaw joint formed by the
quadrate and
articular
The articular bone is part of the lower jaw of most vertebrates, including most jawed fish, amphibians, birds and various kinds of reptiles, as well as ancestral mammals.
Anatomy
In most vertebrates, the articular bone is connected to two oth ...
bones, and certain characteristics of the
vertebrae
The spinal column, a defining synapomorphy shared by nearly all vertebrates, Hagfish are believed to have secondarily lost their spinal column is a moderately flexible series of vertebrae (singular vertebra), each constituting a characteristi ...
. The animals singled out by these formulations, the
amniotes other than the mammals and the birds, are still those considered reptiles today.

The synapsid/sauropsid division supplemented another approach, one that split the reptiles into four subclasses based on the number and position of
temporal fenestrae
The skull is a bone protective cavity for the brain. The skull is composed of four types of bone i.e., cranial bones, facial bones, ear ossicles and hyoid bone. However two parts are more prominent: the cranium and the mandible. In humans, th ...
, openings in the sides of the skull behind the eyes. This classification was initiated by
Henry Fairfield Osborn
Henry Fairfield Osborn, Sr. (August 8, 1857 – November 6, 1935) was an American paleontologist, geologist and eugenics advocate. He was the president of the American Museum of Natural History for 25 years and a cofounder of the American Euge ...
and elaborated and made popular by
Romer
A Reference Card or "Romer" is a device for increasing the accuracy when reading a grid reference from a map. Made from transparent plastic, paper or other materials, they are also found on most baseplate compasses. Essentially, it is a speciall ...
's classic ''
Vertebrate Paleontology
Vertebrate paleontology is the subfield of paleontology that seeks to discover, through the study of fossilized remains, the behavior, reproduction and appearance of extinct animals with vertebrae or a notochord. It also tries to connect, by us ...
''.
[, 3rd ed., 1966.] Those four subclasses were:
*
Anapsid
An anapsid is an amniote whose skull lacks one or more skull openings (fenestra, or fossae) near the temples. Traditionally, the Anapsida are the most primitive subclass of amniotes, the ancestral stock from which Synapsida and Diapsida evolve ...
a – no fenestrae –
cotylosaur
Captorhinidae (also known as cotylosaurs) is an extinct family of tetrapods, traditionally considered primitive reptiles, known from the late Carboniferous to the Late Permian. They had a cosmopolitan distribution across Pangea.
Description
C ...
s and
Chelonia
The green sea turtle (''Chelonia mydas''), also known as the green turtle, black (sea) turtle or Pacific green turtle, is a species of large sea turtle of the family Cheloniidae. It is the only species in the genus ''Chelonia''. Its range exten ...
(
turtle
Turtles are an order of reptiles known as Testudines, characterized by a special shell developed mainly from their ribs. Modern turtles are divided into two major groups, the Pleurodira (side necked turtles) and Cryptodira (hidden necked t ...
s and relatives)
[This taxonomy does not reflect modern molecular evidence, which places turtles within Diapsida.]
*
Synapsid
Synapsids + (, 'arch') > () "having a fused arch"; synonymous with ''theropsids'' (Greek, "beast-face") are one of the two major groups of animals that evolved from basal amniotes, the other being the sauropsids, the group that includes reptil ...
a – one low fenestra –
pelycosaur
Pelycosaur ( ) is an older term for basal or primitive Late Paleozoic synapsids, excluding the therapsids and their descendants. Previously, the term ''mammal-like reptile'' had been used, and pelycosaur was considered an order, but this is ...
s and
therapsid
Therapsida is a major group of eupelycosaurian synapsids that includes mammals, their ancestors and relatives. Many of the traits today seen as unique to mammals had their origin within early therapsids, including limbs that were oriented more ...
s (the '
mammal-like reptiles
Pelycosaur ( ) is an older term for basal or primitive Late Paleozoic synapsids, excluding the therapsids and their descendants. Previously, the term ''mammal-like reptile'' had been used, and pelycosaur was considered an order, but this is ...
')
*
Euryapsida – one high fenestra (above the postorbital and squamosal) –
protorosaurs (small, early lizard-like reptiles) and the marine
sauropterygia
Sauropterygia ("lizard flippers") is an extinct taxon of diverse, aquatic reptiles that developed from terrestrial ancestors soon after the end-Permian extinction and flourished during the Triassic before all except for the Plesiosauria became ...
ns and
ichthyosaurs
Ichthyosaurs (Ancient Greek for "fish lizard" – and ) are large extinct marine reptiles. Ichthyosaurs belong to the order known as Ichthyosauria or Ichthyopterygia ('fish flippers' – a designation introduced by Sir Richard Owen in 1842, alt ...
, the latter called
Parapsida in Osborn's work.
*
Diapsid
Diapsids ("two arches") are a clade of sauropsids, distinguished from more primitive eureptiles by the presence of two holes, known as temporal fenestrae, in each side of their skulls. The group first appeared about three hundred million years a ...
a – two fenestrae – most reptiles, including
lizards,
snake
Snakes are elongated, limbless, carnivorous reptiles of the suborder Serpentes . Like all other squamates, snakes are ectothermic, amniote vertebrates covered in overlapping scales. Many species of snakes have skulls with several more j ...
s,
crocodilians,
dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s and
pterosaur
Pterosaurs (; from Greek ''pteron'' and ''sauros'', meaning "wing lizard") is an extinct clade of flying reptiles in the order, Pterosauria. They existed during most of the Mesozoic: from the Late Triassic to the end of the Cretaceous (228 ...
s
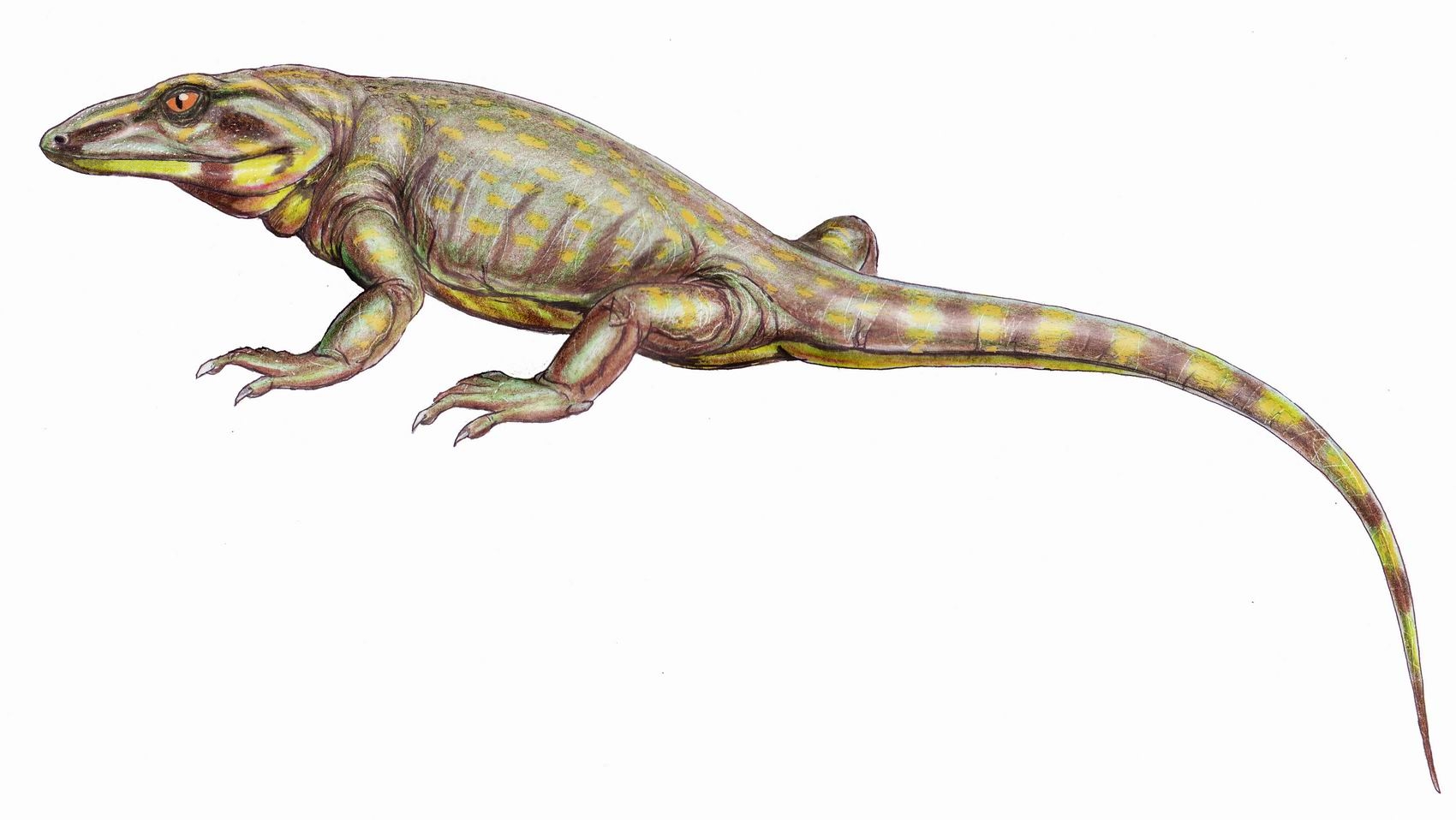
The composition of Euryapsida was uncertain.
Ichthyosaurs
Ichthyosaurs (Ancient Greek for "fish lizard" – and ) are large extinct marine reptiles. Ichthyosaurs belong to the order known as Ichthyosauria or Ichthyopterygia ('fish flippers' – a designation introduced by Sir Richard Owen in 1842, alt ...
were, at times, considered to have arisen independently of the other euryapsids, and given the older name Parapsida. Parapsida was later discarded as a group for the most part (ichthyosaurs being classified as ''
incertae sedis
' () or ''problematica'' is a term used for a taxonomic group where its broader relationships are unknown or undefined. Alternatively, such groups are frequently referred to as "enigmatic taxa". In the system of open nomenclature, uncertain ...
'' or with Euryapsida). However, four (or three if Euryapsida is merged into Diapsida) subclasses remained more or less universal for non-specialist work throughout the 20th century. It has largely been abandoned by recent researchers: In particular, the anapsid condition has been found to occur so variably among unrelated groups that it is not now considered a useful distinction.
Phylogenetics and modern definition
By the early 21st century, vertebrate paleontologists were beginning to adopt
phylogenetic
In biology, phylogenetics (; from Greek φυλή/ φῦλον [] "tribe, clan, race", and wikt:γενετικός, γενετικός [] "origin, source, birth") is the study of the evolutionary history and relationships among or within groups o ...
taxonomy, in which all groups are defined in such a way as to be
monophyletic; that is, groups which include all descendants of a particular ancestor. The reptiles as historically defined are
paraphyletic, since they exclude both birds and mammals. These respectively evolved from dinosaurs and from early therapsids, which were both traditionally called reptiles.
Birds are more closely related to
crocodilians than the latter are to the rest of extant reptiles.
Colin Tudge wrote:
Mammals are a clade, and therefore the cladists are happy to acknowledge the traditional taxon Mammalia; and birds, too, are a clade, universally ascribed to the formal taxon Aves. Mammalia and Aves are, in fact, subclades within the grand clade of the Amniota. But the traditional class Reptilia is not a clade. It is just a section of the clade Amniota
Amniotes are a clade of tetrapod vertebrates that comprises sauropsids (including all reptiles and birds, and extinct parareptiles and non-avian dinosaurs) and synapsids (including pelycosaurs and therapsids such as mammals). They are dis ...
: the section that is left after the Mammalia and Aves have been hived off. It cannot be defined by synapomorphies, as is the proper way. Instead, it is defined by a combination of the features it has and the features it lacks: reptiles are the amniotes that lack fur or feathers. At best, the cladists suggest, we could say that the traditional Reptilia are 'non-avian, non-mammalian amniotes'.
Despite the early proposals for replacing the paraphyletic Reptilia with a monophyletic
Sauropsida, which includes birds, that term was never adopted widely or, when it was, was not applied consistently.

When Sauropsida was used, it often had the same content or even the same definition as Reptilia. In 1988,
Jacques Gauthier proposed a
cladistic
Cladistics (; ) is an approach to biological classification in which organisms are categorized in groups ("clades") based on hypotheses of most recent common ancestry. The evidence for hypothesized relationships is typically shared derived char ...
definition of Reptilia as a monophyletic node-based
crown group containing turtles, lizards and snakes, crocodilians, and birds, their common ancestor and all its descendants. While Gauthier's definition was close to the modern consensus, nonetheless, it became considered inadequate because the actual relationship of turtles to other reptiles was not yet well understood at this time.
[ Major revisions since have included the reassignment of synapsids as non-reptiles, and classification of turtles as diapsids.]PhyloCode
The ''International Code of Phylogenetic Nomenclature'', known as the ''PhyloCode'' for short, is a formal set of rules governing phylogenetic nomenclature. Its current version is specifically designed to regulate the naming of clades, leaving the ...
, was published by Modesto and Anderson in 2004. Modesto and Anderson reviewed the many previous definitions and proposed a modified definition, which they intended to retain most traditional content of the group while keeping it stable and monophyletic. They defined Reptilia as all amniotes closer to ''Lacerta agilis
The sand lizard (''Lacerta agilis'') is a Lacertidae, lacertid lizard distributed across most of Europe from France and across the continent to Lake Baikal in Russia. It does not occur in European Turkey. Its distribution is often patchy. In the ...
'' and ''Crocodylus niloticus
''Crocodylus'' is a genus of true crocodiles in the family Crocodylidae.
Taxonomy
The generic name, ''Crocodylus'', was proposed by Josephus Nicolaus Laurenti in 1768. ''Crocodylus'' contains 13–14 extant (living) species and 5 extinct spe ...
'' than to ''Homo sapiens
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, culture, ...
''. This stem-based definition is equivalent to the more common definition of Sauropsida, which Modesto and Anderson synonymized with Reptilia, since the latter is better known and more frequently used. Unlike most previous definitions of Reptilia, however, Modesto and Anderson's definition includes birds,[ as they are within the clade that includes both lizards and crocodiles.]
Taxonomy
General classification of extinct and living reptiles, focusing on major groups.Parareptilia
Parareptilia ("at the side of reptiles") is a subclass or clade of basal sauropsids (reptiles), typically considered the sister taxon to Eureptilia (the group that likely contains all living reptiles and birds). Parareptiles first arose near th ...
** Eureptilia
Eureptilia ("true reptiles") is one of the two major subgroups of the clade Sauropsida, the other one being Parareptilia. Eureptilia includes Diapsida (the clade containing all modern reptiles and birds), as well as a number of primitive Permo ...
***Captorhinidae
Captorhinidae (also known as cotylosaurs) is an extinct family of tetrapods, traditionally considered primitive reptiles, known from the late Carboniferous to the Late Permian. They had a cosmopolitan distribution across Pangea.
Description
...
***Diapsid
Diapsids ("two arches") are a clade of sauropsids, distinguished from more primitive eureptiles by the presence of two holes, known as temporal fenestrae, in each side of their skulls. The group first appeared about three hundred million years a ...
a
****Araeoscelidia
Araeoscelidia or Araeoscelida is a clade of extinct diapsid reptiles superficially resembling lizards, extending from the Late Carboniferous to the Early Permian.
The group contains the genera ''Araeoscelis'', ''Petrolacosaurus'', the possi ...
****Neodiapsida
Neodiapsida is a clade, or major branch, of the reptilian family tree, typically defined as including all diapsids apart from some early primitive types known as the araeoscelidians. Modern reptiles and birds belong to the neodiapsid subclade S ...
***** Drepanosauromorpha (placement uncertain)
*****Younginiformes
Younginiformes is a replacement name for the taxon Eosuchia, proposed by Alfred Romer in 1947.
The Eosuchia having become a wastebasket taxon for many probably distantly-related primitive diapsid reptiles ranging from the late Carboniferous to ...
( paraphyletic)
*****Ichthyosauromorpha
The Ichthyosauromorpha are an extinct clade of marine reptiles consisting of the Ichthyosauriformes and the Hupehsuchia, living during the Mesozoic.
The node clade Ichthyosauromorpha was first defined by Ryosuke Motani ''et al.'' in 2014 as t ...
(placement uncertain)
***** Thalattosauria (placement uncertain)
*****Sauria
Sauria is the clade containing the most recent common ancestor of archosaurs (such as crocodilians, dinosaurs, etc.) and lepidosaurs ( lizards and kin), and all its descendants. Since most molecular phylogenies recover turtles as more closely ...
****** Lepidosauromorpha
******* Lepidosauriformes
******** Rhynchocephalia (tuatara)
********Squamata
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species ...
(lizards & snakes)
****** Choristodera (placement uncertain)
******Sauropterygia
Sauropterygia ("lizard flippers") is an extinct taxon of diverse, aquatic reptiles that developed from terrestrial ancestors soon after the end-Permian extinction and flourished during the Triassic before all except for the Plesiosauria became ...
(placement uncertain)
******Pantestudines
Pantestudines or Pan-Testudines is the group of all reptiles more closely related to turtles than to any other living animal. It includes both modern turtles (crown group turtles, also known as Testudines) and all of their extinct relatives (also ...
(turtles and kin, placement uncertain)
****** Archosauromorpha
*******Protorosauria
Protorosauria is an extinct polyphyletic group of archosauromorph reptiles from the latest Middle Permian ( Capitanian stage) to the end of the Late Triassic (Rhaetian stage) of Asia, Europe and North America. It was named by the English anatom ...
(paraphyletic)
*******Rhynchosauria
Rhynchosaurs are a group of extinct herbivorous Triassic archosauromorph reptiles, belonging to the order Rhynchosauria. Members of the group are distinguished by their triangular skulls and elongated, beak like premaxillary bones. Rhynchosaurs ...
*******Allokotosauria
Allokotosauria is a clade of early archosauromorph reptiles from the Middle to Late Triassic known from Asia, Africa, North America and Europe. Allokotosauria was first described and named when a new monophyletic grouping of specialized he ...
*******Archosauriformes
Archosauriformes (Greek for 'ruling lizards', and Latin for 'form') is a clade of diapsid reptiles that developed from archosauromorph ancestors some time in the Latest Permian (roughly 252 million years ago). It was defined by Jacques Gauthier ...
********Phytosaur
Phytosaurs (Φυτόσαυροι in greek) are an extinct group of large, mostly semiaquatic Late Triassic archosauriform reptiles. Phytosaurs belong to the order Phytosauria. Phytosauria and Phytosauridae are often considered to be equivalent g ...
ia
******** Archosauria
*********Pseudosuchia
Pseudosuchia is one of two major divisions of Archosauria, including living crocodilians and all archosaurs more closely related to crocodilians than to birds. Pseudosuchians are also informally known as "crocodilian-line archosaurs". Prior to ...
**********Crocodilia
Crocodilia (or Crocodylia, both ) is an order of mostly large, predatory, semiaquatic reptiles, known as crocodilians. They first appeared 95 million years ago in the Late Cretaceous period ( Cenomanian stage) and are the closest livi ...
(crocodilians)
*********Avemetatarsalia
Avemetatarsalia (meaning "bird metatarsals") is a clade of diapsid reptiles containing all archosaurs more closely related to birds than to crocodilians. The two most successful groups of avemetatarsalians were the dinosaurs and pterosaurs. ...
/Ornithodira
Avemetatarsalia (meaning "bird metatarsals") is a clade of diapsid reptiles containing all archosaurs more closely related to birds than to crocodilians. The two most successful groups of avemetatarsalians were the dinosaurs and pterosaurs. Dinos ...
**********Pterosaur
Pterosaurs (; from Greek ''pteron'' and ''sauros'', meaning "wing lizard") is an extinct clade of flying reptiles in the order, Pterosauria. They existed during most of the Mesozoic: from the Late Triassic to the end of the Cretaceous (228 ...
ia
**********Dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
ia
*********** Ornithischia
***********Saurischia
Saurischia ( , meaning "reptile-hipped" from the Greek ' () meaning 'lizard' and ' () meaning 'hip joint') is one of the two basic divisions of dinosaurs (the other being Ornithischia), classified by their hip structure. Saurischia and Ornithis ...
(including birds ( Aves))
Phylogeny
The cladogram
A cladogram (from Greek ''clados'' "branch" and ''gramma'' "character") is a diagram used in cladistics to show relations among organisms. A cladogram is not, however, an evolutionary tree because it does not show how ancestors are related to ...
presented here illustrates the "family tree" of reptiles, and follows a simplified version of the relationships found by M.S. Lee, in 2013.[
]
The position of turtles
The placement of turtles has historically been highly variable. Classically, turtles were considered to be related to the primitive anapsid reptiles.organogenesis
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation (the ectoderm, endoderm, and mesoderm) form the internal org ...
, Werneburg and Sánchez-Villagra (2009) found support for the hypothesis that turtles belong to a separate clade within Sauropsida, outside the sauria
Sauria is the clade containing the most recent common ancestor of archosaurs (such as crocodilians, dinosaurs, etc.) and lepidosaurs ( lizards and kin), and all its descendants. Since most molecular phylogenies recover turtles as more closely ...
n clade altogether.
Evolutionary history
Origin of the reptiles

 The origin of the reptiles lies about 310–320 million years ago, in the steaming swamps of the late Carboniferous period, when the first reptiles evolved from advanced reptiliomorphs.
The origin of the reptiles lies about 310–320 million years ago, in the steaming swamps of the late Carboniferous period, when the first reptiles evolved from advanced reptiliomorphs.[
]
The oldest known animal that may have been an amniote is ''Casineria
''Casineria'' is an extinct genus of tetrapod which lived about 340-334 million years ago in the Mississippian epoch of the Carboniferous period. Its generic name, ''Casineria'', is a latinization of Cheese Bay, the site near Edinburgh, Scotla ...
'' (though it may have been a temnospondyl). A series of footprints from the fossil strata of Nova Scotia
Nova Scotia ( ; ; ) is one of the thirteen provinces and territories of Canada. It is one of the three Maritime provinces and one of the four Atlantic provinces. Nova Scotia is Latin for "New Scotland".
Most of the population are native Eng ...
dated to show typical reptilian toes and imprints of scales. These tracks are attributed to ''Hylonomus
''Hylonomus'' (; ''hylo-'' "forest" + ''nomos'' "dweller") is an extinct genus of reptile that lived 312 million years ago during the Late Carboniferous period.
It is the earliest unquestionable reptile (''Westlothiana'' is older, but in fact it ...
'', the oldest unquestionable reptile known.
It was a small, lizard-like animal, about long, with numerous sharp teeth indicating an insectivorous diet.Westlothiana
''Westlothiana'' ("animal from West Lothian") is a genus of reptile-like tetrapod that lived about 338 million years ago during the latest part of the Visean age of the Carboniferous. Members of the genus bore a superficial resemblance to modern ...
'' (for the moment considered a reptiliomorph
Reptiliomorpha (meaning reptile-shaped; in PhyloCode known as ''Pan-Amniota'') is a clade containing the amniotes and those tetrapods that share a more recent common ancestor with amniotes than with living amphibians (lissamphibians). It was defi ...
rather than a true amniote) and '' Paleothyris'', both of similar build and presumably similar habit.
However, microsaur
Microsauria ("small lizards") is an extinct, possibly polyphyletic order of tetrapods from the late Carboniferous and early Permian periods. It is the most diverse and species-rich group of lepospondyls. Recently, Microsauria has been consider ...
s have been at times considered true reptiles, so an earlier origin is possible.
Rise of the reptiles
The earliest amniotes, including stem-reptiles (those amniotes closer to modern reptiles than to mammals), were largely overshadowed by larger stem-tetrapods, such as '' Cochleosaurus'', and remained a small, inconspicuous part of the fauna until the Carboniferous Rainforest Collapse.Mesosaurus
''Mesosaurus'' (meaning "middle lizard") is an extinct genus of reptile from the Cisuralian, Early Permian of southern Africa and South America. Along with it, the genera ''Brazilosaurus'' and ''Stereosternum'', it is a member of the family (bio ...
'', a genus from the Early Permian that had returned to water, feeding on fish.
A 2021 examination of reptile diversity in the Carboniferous and Permian suggests a much higher degree of diversity than previously thought, comparable or even exceeding that of synapsids. Thus, the "First Age of Reptiles" was proposed.
Anapsids, synapsids, diapsids, and sauropsids
 It was traditionally assumed that the first reptiles retained an
It was traditionally assumed that the first reptiles retained an anapsid
An anapsid is an amniote whose skull lacks one or more skull openings (fenestra, or fossae) near the temples. Traditionally, the Anapsida are the most primitive subclass of amniotes, the ancestral stock from which Synapsida and Diapsida evolve ...
skull inherited from their ancestors.[Coven, R (2000): History of Life. ]Blackwell Science
Wiley-Blackwell is an international scientific, technical, medical, and scholarly publishing business of John Wiley & Sons. It was formed by the merger of John Wiley & Sons Global Scientific, Technical, and Medical business with Blackwell Publish ...
, Oxford, UK
p 154
from Google Books This type of skull has a skull roof with only holes for the nostrils, eyes and a pineal eye.[ Romer, A.S. & T.S. Parsons. 1977. ''The Vertebrate Body.'' 5th ed. Saunders, Philadelphia. (6th ed. 1985)] The discoveries of synapsid
Synapsids + (, 'arch') > () "having a fused arch"; synonymous with ''theropsids'' (Greek, "beast-face") are one of the two major groups of animals that evolved from basal amniotes, the other being the sauropsids, the group that includes reptil ...
-like openings (see below) in the skull roof of the skulls of several members of Parareptilia
Parareptilia ("at the side of reptiles") is a subclass or clade of basal sauropsids (reptiles), typically considered the sister taxon to Eureptilia (the group that likely contains all living reptiles and birds). Parareptiles first arose near th ...
(the clade containing most of the amniotes traditionally referred to as "anapsids"), including lanthanosuchoids, millerettids, bolosaurids, some nycteroleterids, some procolophonoids and at least some mesosaur
Mesosaurs ("middle lizards") were a group of small aquatic reptiles that lived during the early Permian period, roughly 299 to 270 million years ago. Mesosaurs were the first known aquatic reptiles, having apparently returned to an aquatic life ...
s[ Very shortly after the first amniotes appeared, a lineage called Synapsida split off; this group was characterized by a temporal opening in the skull behind each eye to give room for the jaw muscle to move. These are the "mammal-like amniotes", or stem-mammals, that later gave rise to the true ]mammals
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur o ...
. Soon after, another group evolved a similar trait, this time with a double opening behind each eye, earning them the name Diapsida
Diapsids ("two arches") are a clade of sauropsids, distinguished from more primitive eureptiles by the presence of two holes, known as temporal fenestrae, in each side of their skulls. The group first appeared about three hundred million years ag ...
("two arches").[ The function of the holes in these groups was to lighten the skull and give room for the jaw muscles to move, allowing for a more powerful bite.]phylogenetic
In biology, phylogenetics (; from Greek φυλή/ φῦλον [] "tribe, clan, race", and wikt:γενετικός, γενετικός [] "origin, source, birth") is the study of the evolutionary history and relationships among or within groups o ...
studies with this in mind placed turtles firmly within Diapsida.
Permian reptiles
With the close of the Carboniferous, the amniotes became the dominant tetrapod fauna. While primitive, terrestrial reptiliomorphs still existed, the synapsid amniotes evolved the first truly terrestrial megafauna (giant animals) in the form of pelycosaurs
Pelycosaur ( ) is an older term for basal or primitive Late Paleozoic synapsids, excluding the therapsids and their descendants. Previously, the term ''mammal-like reptile'' had been used, and pelycosaur was considered an order, but this is now ...
, such as ''Edaphosaurus
''Edaphosaurus'' (, meaning "pavement lizard" for dense clusters of teeth) is a genus of extinct edaphosaurid synapsids that lived in what is now North America and Europe around 303.4 to 272.5 million years ago, during the Late Carboniferous to ...
'' and the carnivorous '' Dimetrodon''. In the mid-Permian period, the climate became drier, resulting in a change of fauna: The pelycosaurs were replaced by the therapsids
Therapsida is a major group of eupelycosaurian synapsids that includes mammals, their ancestors and relatives. Many of the traits today seen as unique to mammals had their origin within early therapsids, including limbs that were oriented mo ...
.[ Colbert, E.H. & Morales, M. (2001): '' Colbert's Evolution of the Vertebrates: A History of the Backboned Animals Through Time''. 4th edition. John Wiley & Sons, Inc, New York. .]
The parareptiles, whose massive skull roofs had no postorbital holes, continued and flourished throughout the Permian. The pareiasaurian parareptiles reached giant proportions in the late Permian, eventually disappearing at the close of the period (the turtles being possible survivors).turtle
Turtles are an order of reptiles known as Testudines, characterized by a special shell developed mainly from their ribs. Modern turtles are divided into two major groups, the Pleurodira (side necked turtles) and Cryptodira (hidden necked t ...
s, crocodiles, and dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s) and the Lepidosauromorpha (predecessors of modern lizards and tuatara
Tuatara (''Sphenodon punctatus'') are reptiles endemic to New Zealand. Despite their close resemblance to lizards, they are part of a distinct lineage, the order Rhynchocephalia. The name ''tuatara'' is derived from the Māori language and m ...
s). Both groups remained lizard-like and relatively small and inconspicuous during the Permian.
Mesozoic reptiles
The close of the Permian saw the greatest mass extinction known (see the Permian–Triassic extinction event
The Permian–Triassic (P–T, P–Tr) extinction event, also known as the Latest Permian extinction event, the End-Permian Extinction and colloquially as the Great Dying, formed the boundary between the Permian and Triassic geologic periods, as ...
), an event prolonged by the combination of two or more distinct extinction pulses.Triassic
The Triassic ( ) is a geologic period and system (stratigraphy), system which spans 50.6 million years from the end of the Permian Period 251.902 million years ago (Year#Abbreviations yr and ya, Mya), to the beginning of the Jurassic Period 201.36 ...
period, though it took 30 million years before their diversity was as great as the animals that lived in the Permian.dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s and pterosaur
Pterosaurs (; from Greek ''pteron'' and ''sauros'', meaning "wing lizard") is an extinct clade of flying reptiles in the order, Pterosauria. They existed during most of the Mesozoic: from the Late Triassic to the end of the Cretaceous (228 ...
s, as well as the ancestors of crocodiles. Since reptiles, first rauisuchia
"Rauisuchia" is a paraphyletic group of mostly large and carnivorous Triassic archosaurs. Rauisuchians are a category of archosaurs within a larger group called Pseudosuchia, which encompasses all archosaurs more closely related to crocodilians ...
ns and then dinosaurs, dominated the Mesozoic era, the interval is popularly known as the "Age of Reptiles". The dinosaurs also developed smaller forms, including the feather-bearing smaller theropods
Theropoda (; ), whose members are known as theropods, is a dinosaur clade that is characterized by hollow bones and three toes and claws on each limb. Theropods are generally classed as a group of saurischian dinosaurs. They were ancestrally ca ...
. In the Cretaceous
The Cretaceous ( ) is a geological period that lasted from about 145 to 66 million years ago (Mya). It is the third and final period of the Mesozoic Era, as well as the longest. At around 79 million years, it is the longest geological period of ...
period, these gave rise to the first true birds.tuatara
Tuatara (''Sphenodon punctatus'') are reptiles endemic to New Zealand. Despite their close resemblance to lizards, they are part of a distinct lineage, the order Rhynchocephalia. The name ''tuatara'' is derived from the Māori language and m ...
s, as well as their fossil relatives. Lepidosauromorpha contained at least one major group of the Mesozoic sea reptiles: the mosasaurs, which lived during the Cretaceous
The Cretaceous ( ) is a geological period that lasted from about 145 to 66 million years ago (Mya). It is the third and final period of the Mesozoic Era, as well as the longest. At around 79 million years, it is the longest geological period of ...
period. The phylogenetic placement of other main groups of fossil sea reptiles – the ichthyopterygia
Ichthyopterygia ("fish flippers") was a designation introduced by Sir Richard Owen in 1840 to designate the Jurassic ichthyosaurs that were known at the time, but the term is now used more often for both true Ichthyosauria and their more primitiv ...
ns (including ichthyosaurs) and the sauropterygia
Sauropterygia ("lizard flippers") is an extinct taxon of diverse, aquatic reptiles that developed from terrestrial ancestors soon after the end-Permian extinction and flourished during the Triassic before all except for the Plesiosauria became ...
ns, which evolved in the early Triassic – is more controversial. Different authors linked these groups either to lepidosauromorphs[Gauthier J.A. (1994): ''The diversification of the amniotes''. In: D.R. Prothero and R.M. Schoch (ed.) Major Features of Vertebrate Evolution: 129–159. Knoxville, Tennessee: The Paleontological Society.] or to archosauromorphs, and ichthyopterygians were also argued to be diapsids that did not belong to the least inclusive clade containing lepidosauromorphs and archosauromorphs.
Cenozoic reptiles

 The close of the
The close of the Cretaceous
The Cretaceous ( ) is a geological period that lasted from about 145 to 66 million years ago (Mya). It is the third and final period of the Mesozoic Era, as well as the longest. At around 79 million years, it is the longest geological period of ...
period saw the demise of the Mesozoic era reptilian megafauna (see the Cretaceous–Paleogene extinction event
The Cretaceous–Paleogene (K–Pg) extinction event (also known as the Cretaceous–Tertiary extinction) was a sudden mass extinction of three-quarters of the plant and animal species on Earth, approximately 66 million years ago. With the ...
, also known as K-T extinction event). Of the large marine reptiles, only sea turtle
Sea turtles (superfamily Chelonioidea), sometimes called marine turtles, are reptiles of the order Testudines and of the suborder Cryptodira. The seven existing species of sea turtles are the flatback, green, hawksbill, leatherback, loggerhe ...
s were left; and of the non-marine large reptiles, only the semi-aquatic crocodiles
Crocodiles (family Crocodylidae) or true crocodiles are large semiaquatic reptiles that live throughout the tropics in Africa, Asia, the Americas and Australia. The term crocodile is sometimes used even more loosely to include all extant memb ...
and broadly similar choristoderes survived the extinction, with last members of the latter, the lizard-like '' Lazarussuchus'', becoming extinct in the Miocene
The Miocene ( ) is the first epoch (geology), geological epoch of the Neogene Period and extends from about (Ma). The Miocene was named by Scottish geologist Charles Lyell; the name comes from the Greek words (', "less") and (', "new") and mea ...
. Of the great host of dinosaurs dominating the Mesozoic, only the small beaked birds survived. This dramatic extinction pattern at the end of the Mesozoic led into the Cenozoic. Mammals and birds filled the empty niches left behind by the reptilian megafauna and, while reptile diversification slowed, bird and mammal diversification took an exponential turn.tortoise
Tortoises () are reptiles of the family Testudinidae of the order Testudines (Latin: ''tortoise''). Like other turtles, tortoises have a shell to protect from predation and other threats. The shell in tortoises is generally hard, and like oth ...
s.Squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
took a massive hit during the K–Pg event, only recovering ten million years after it,domesticated
Domestication is a sustained multi-generational relationship in which humans assume a significant degree of control over the reproduction and care of another group of organisms to secure a more predictable supply of resources from that group. A ...
species).
Morphology and physiology
Circulation
 All lepidosaurs and
All lepidosaurs and turtle
Turtles are an order of reptiles known as Testudines, characterized by a special shell developed mainly from their ribs. Modern turtles are divided into two major groups, the Pleurodira (side necked turtles) and Cryptodira (hidden necked t ...
s have a three-chambered heart
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to t ...
consisting of two atria, one variably partitioned ventricle, and two aortas that lead to the systemic circulation. The degree of mixing of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
ated and deoxygenated blood in the three-chambered heart varies depending on the species and physiological state. Under different conditions, deoxygenated blood can be shunted back to the body or oxygenated blood can be shunted back to the lungs. This variation in blood flow has been hypothesized to allow more effective thermoregulation and longer diving times for aquatic species, but has not been shown to be a fitness advantage.
 For example,
For example, Iguana
''Iguana'' (, ) is a genus of herbivorous lizards that are native to tropical areas of Mexico, Central America, South America, and the Caribbean. The genus was first described in 1768 by Austrian naturalist Josephus Nicolaus Laurenti in his ...
hearts, like the majority of the squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
hearts, are composed of three chambers with two aorta and one ventricle, cardiac involuntary muscles. The main structures of the heart are the sinus venosus
The sinus venosus is a large quadrangular cavity which precedes the atrium on the venous side of the chordate heart. In mammals, it exists distinctly only in the embryonic heart, where it is found between the two venae cavae. However, the sinus v ...
, the pacemaker, the left atrium
The atrium ( la, ātrium, , entry hall) is one of two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular valves.
There are two at ...
, the right atrium
The atrium ( la, ātrium, , entry hall) is one of two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular valves.
There are two at ...
, the atrioventricular valve, the cavum venosum, cavum arteriosum, the cavum pulmonale, the muscular ridge, the ventricular ridge, pulmonary vein
The pulmonary veins are the veins that transfer oxygenated blood from the lungs to the heart. The largest pulmonary veins are the four ''main pulmonary veins'', two from each lung that drain into the left atrium of the heart. The pulmonary vei ...
s, and paired aortic arches
The aortic arches or pharyngeal arch arteries (previously referred to as branchial arches in human embryos) are a series of six paired embryological vascular structures which give rise to the great arteries of the neck and head. They are ventral ...
.
Some squamate species (e.g., pythons and monitor lizards) have three-chambered hearts that become functionally four-chambered hearts during contraction. This is made possible by a muscular ridge that subdivides the ventricle during ventricular diastole and completely divides it during ventricular systole. Because of this ridge, some of these squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
are capable of producing ventricular pressure differentials that are equivalent to those seen in mammalian and avian hearts.
Crocodilia
Crocodilia (or Crocodylia, both ) is an order of mostly large, predatory, semiaquatic reptiles, known as crocodilians. They first appeared 95 million years ago in the Late Cretaceous period ( Cenomanian stage) and are the closest livi ...
ns have an anatomically four-chambered heart, similar to bird
Birds are a group of warm-blooded vertebrates constituting the class Aves (), characterised by feathers, toothless beaked jaws, the laying of hard-shelled eggs, a high metabolic rate, a four-chambered heart, and a strong yet lightweig ...
s, but also have two systemic aortas and are therefore capable of bypassing their pulmonary circulation
The pulmonary circulation is a division of the circulatory system in all vertebrates. The circuit begins with deoxygenated blood returned from the body to the right atrium of the heart where it is pumped out from the right ventricle to the lungs ...
.
Metabolism
 Modern non-avian reptiles exhibit some form of cold-bloodedness (i.e. some mix of
Modern non-avian reptiles exhibit some form of cold-bloodedness (i.e. some mix of poikilotherm
A poikilotherm () is an animal whose internal temperature varies considerably. Poikilotherms have to survive and adapt to environmental stress. One of the most important stressors is temperature change, which can lead to alterations in membrane ...
y, ectotherm
An ectotherm (from the Greek () "outside" and () "heat") is an organism in which internal physiological sources of heat are of relatively small or of quite negligible importance in controlling body temperature.Davenport, John. Animal Life ...
y, and bradymetabolism
Bradymetabolism refers to organisms with a high active metabolism and a considerably slower resting metabolism.Bligh, J., and Johnson, K.G., 1973. Glossary for terms for thermal physiology. ''Journal of Applied Physiology'' 35(6):941–961. Bradym ...
) so that they have limited physiological means of keeping the body temperature constant and often rely on external sources of heat. Due to a less stable core temperature than bird
Birds are a group of warm-blooded vertebrates constituting the class Aves (), characterised by feathers, toothless beaked jaws, the laying of hard-shelled eggs, a high metabolic rate, a four-chambered heart, and a strong yet lightweig ...
s and mammals, reptilian biochemistry requires enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s capable of maintaining efficiency over a greater range of temperatures than in the case for warm-blooded
Warm-blooded is an informal term referring to animal species which can maintain a body temperature higher than their environment. In particular, homeothermic species maintain a stable body temperature by regulating metabolic processes. The onl ...
animals. The optimum body temperature range varies with species, but is typically below that of warm-blooded animals; for many lizards, it falls in the 24°–35 °C (75°–95 °F) range, while extreme heat-adapted species, like the American desert iguana
The desert iguana (''Dipsosaurus dorsalis'') is an iguana species found in the Sonoran and Mojave Deserts of the Southwestern United States and northwestern Mexico, as well as on several Gulf of California islands.
Taxonomy
The species was ...
''Dipsosaurus dorsalis'', can have optimal physiological temperatures in the mammalian range, between 35° and 40 °C (95° and 104 °F). While the optimum temperature is often encountered when the animal is active, the low basal metabolism makes body temperature drop rapidly when the animal is inactive.
As in all animals, reptilian muscle action produces heat. In large reptiles, like leatherback turtles, the low surface-to-volume ratio allows this metabolically produced heat to keep the animals warmer than their environment even though they do not have a warm-blooded
Warm-blooded is an informal term referring to animal species which can maintain a body temperature higher than their environment. In particular, homeothermic species maintain a stable body temperature by regulating metabolic processes. The onl ...
metabolism. This form of homeothermy is called gigantothermy Gigantothermy (sometimes called ectothermic homeothermy or inertial homeothermy) is a phenomenon with significance in biology and paleontology, whereby large, bulky ectothermic animals are more easily able to maintain a constant, relatively high bod ...
; it has been suggested as having been common in large dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s and other extinct large-bodied reptiles.
The benefit of a low resting metabolism is that it requires far less fuel to sustain bodily functions. By using temperature variations in their surroundings, or by remaining cold when they do not need to move, reptiles can save considerable amounts of energy compared to endothermic animals of the same size. A crocodile needs from a tenth to a fifth of the food necessary for a lion of the same weight and can live half a year without eating.warm-blooded
Warm-blooded is an informal term referring to animal species which can maintain a body temperature higher than their environment. In particular, homeothermic species maintain a stable body temperature by regulating metabolic processes. The onl ...
ness in birds and mammals. However, investigation of correlations between active capacity and thermophysiology show a weak relationship.
Respiratory system
All reptiles breathe using lungs. Aquatic turtle
Turtles are an order of reptiles known as Testudines, characterized by a special shell developed mainly from their ribs. Modern turtles are divided into two major groups, the Pleurodira (side necked turtles) and Cryptodira (hidden necked t ...
s have developed more permeable skin, and some species have modified their cloaca to increase the area for gas exchange
Gas exchange is the physical process by which gases move passively by diffusion across a surface. For example, this surface might be the air/water interface of a water body, the surface of a gas bubble in a liquid, a gas-permeable membrane, or a ...
. Even with these adaptations, breathing is never fully accomplished without lungs. Lung ventilation is accomplished differently in each main reptile group. In squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
, the lungs are ventilated almost exclusively by the axial musculature. This is also the same musculature that is used during locomotion. Because of this constraint, most squamates are forced to hold their breath during intense runs. Some, however, have found a way around it. Varanids, and a few other lizard species, employ buccal pumping
Buccal pumping is "breathing with one's cheeks": a method of ventilation used in respiration in which the animal moves the floor of its mouth in a rhythmic manner that is externally apparent.Brainerd, E. L. (1999). New perspectives on the evolutio ...
as a complement to their normal "axial breathing". This allows the animals to completely fill their lungs during intense locomotion, and thus remain aerobically active for a long time. Tegu lizards are known to possess a proto- diaphragm, which separates the pulmonary cavity from the visceral cavity. While not actually capable of movement, it does allow for greater lung inflation, by taking the weight of the viscera off the lungs.
Crocodilia
Crocodilia (or Crocodylia, both ) is an order of mostly large, predatory, semiaquatic reptiles, known as crocodilians. They first appeared 95 million years ago in the Late Cretaceous period ( Cenomanian stage) and are the closest livi ...
ns actually have a muscular diaphragm that is analogous to the mammalian diaphragm. The difference is that the muscles for the crocodilian diaphragm pull the pubis (part of the pelvis, which is movable in crocodilians) back, which brings the liver down, thus freeing space for the lungs to expand. This type of diaphragmatic setup has been referred to as the " hepatic piston". The airways form a number of double tubular chambers within each lung. On inhalation and exhalation air moves through the airways in the same direction, thus creating a unidirectional airflow through the lungs. A similar system is found in birds, monitor lizards and iguanas.
Most reptiles lack a secondary palate
The secondary palate is an anatomical structure that divides the nasal cavity from the oral cavity in many vertebrates.
In human embryology, it refers to that portion of the hard palate that is formed by the growth of the two palatine shelves medi ...
, meaning that they must hold their breath while swallowing. Crocodilians have evolved a bony secondary palate that allows them to continue breathing while remaining submerged (and protect their brains against damage by struggling prey). Skinks (family Scincidae) also have evolved a bony secondary palate, to varying degrees. Snakes took a different approach and extended their trachea instead. Their tracheal extension sticks out like a fleshy straw, and allows these animals to swallow large prey without suffering from asphyxiation.
Turtles and tortoises
 How turtles and tortoises breathe has been the subject of much study. To date, only a few species have been studied thoroughly enough to get an idea of how those turtles breathe. The varied results indicate that turtles and tortoises have found a variety of solutions to this problem.
The difficulty is that most turtle shells are rigid and do not allow for the type of expansion and contraction that other amniotes use to ventilate their lungs. Some turtles, such as the Indian flapshell ('' Lissemys punctata''), have a sheet of muscle that envelops the lungs. When it contracts, the turtle can exhale. When at rest, the turtle can retract the limbs into the body cavity and force air out of the lungs. When the turtle protracts its limbs, the pressure inside the lungs is reduced, and the turtle can suck air in. Turtle lungs are attached to the inside of the top of the shell (carapace), with the bottom of the lungs attached (via connective tissue) to the rest of the viscera. By using a series of special muscles (roughly equivalent to a diaphragm), turtles are capable of pushing their viscera up and down, resulting in effective respiration, since many of these muscles have attachment points in conjunction with their forelimbs (indeed, many of the muscles expand into the limb pockets during contraction).
Breathing during locomotion has been studied in three species, and they show different patterns. Adult female green sea turtles do not breathe as they crutch along their nesting beaches. They hold their breath during terrestrial locomotion and breathe in bouts as they rest. North American box turtles breathe continuously during locomotion, and the ventilation cycle is not coordinated with the limb movements.
How turtles and tortoises breathe has been the subject of much study. To date, only a few species have been studied thoroughly enough to get an idea of how those turtles breathe. The varied results indicate that turtles and tortoises have found a variety of solutions to this problem.
The difficulty is that most turtle shells are rigid and do not allow for the type of expansion and contraction that other amniotes use to ventilate their lungs. Some turtles, such as the Indian flapshell ('' Lissemys punctata''), have a sheet of muscle that envelops the lungs. When it contracts, the turtle can exhale. When at rest, the turtle can retract the limbs into the body cavity and force air out of the lungs. When the turtle protracts its limbs, the pressure inside the lungs is reduced, and the turtle can suck air in. Turtle lungs are attached to the inside of the top of the shell (carapace), with the bottom of the lungs attached (via connective tissue) to the rest of the viscera. By using a series of special muscles (roughly equivalent to a diaphragm), turtles are capable of pushing their viscera up and down, resulting in effective respiration, since many of these muscles have attachment points in conjunction with their forelimbs (indeed, many of the muscles expand into the limb pockets during contraction).
Breathing during locomotion has been studied in three species, and they show different patterns. Adult female green sea turtles do not breathe as they crutch along their nesting beaches. They hold their breath during terrestrial locomotion and breathe in bouts as they rest. North American box turtles breathe continuously during locomotion, and the ventilation cycle is not coordinated with the limb movements.[
]
Sound production
Compared with frogs, birds, and mammals, reptiles are less vocal. Sound production is usually limited to hissing, which is produced merely by forcing air though a partly closed glottis and is not considered to be a true vocalization. The ability to vocalize exists in crocodilians, some lizards and turtles; and typically involves vibrating fold-like structures in the larynx or glottis. Some geckos and turtles possess true vocal cord
In humans, vocal cords, also known as vocal folds or voice reeds, are folds of throat tissues that are key in creating sounds through vocalization. The size of vocal cords affects the pitch of voice. Open when breathing and vibrating for speec ...
s, which have elastin-rich connective tissue.
Hearing in snakes
Hearing in humans relies on 3 parts of the ear; the outer ear that directs sound waves into the ear canal, the middle ear that transmits incoming sound waves to the inner ear, and the inner ear that helps in hearing and keeping your balance. Unlike humans and other animals, snakes do not possess an outer ear, a middle ear, and a tympanum but have an inner ear structure with cochleas directly connected to their jawbone. They are able to feel the vibrations generated from the sound waves in their jaw as they move on the ground. This is done by the use of mechanoreceptors, sensory nerves that run along the body of snakes directing the vibrations along the spinal nerves to the brain. Snakes have a sensitive auditory perception and can tell which direction sound being made is coming from so that they can sense the presence of prey or predator but it is still unclear how sensitive snakes are to sound waves traveling through the air.
Skin
 Reptilian skin is covered in a horny epidermis, making it watertight and enabling reptiles to live on dry land, in contrast to amphibians. Compared to mammalian skin, that of reptiles is rather thin and lacks the thick
Reptilian skin is covered in a horny epidermis, making it watertight and enabling reptiles to live on dry land, in contrast to amphibians. Compared to mammalian skin, that of reptiles is rather thin and lacks the thick dermal
The dermis or corium is a layer of skin between the epidermis (with which it makes up the cutis) and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided ...
layer that produces leather
Leather is a strong, flexible and durable material obtained from the tanning, or chemical treatment, of animal skins and hides to prevent decay. The most common leathers come from cattle, sheep, goats, equine animals, buffalo, pigs and hog ...
in mammals.
Exposed parts of reptiles are protected by scales or scutes
A scute or scutum (Latin: ''scutum''; plural: ''scuta'' " shield") is a bony external plate or scale overlaid with horn, as on the shell of a turtle, the skin of crocodilians, and the feet of birds. The term is also used to describe the anterio ...
, sometimes with a bony base (osteoderm
Osteoderms are bony deposits forming scales, plates, or other structures based in the dermis. Osteoderms are found in many groups of extant and extinct reptiles and amphibians, including lizards, crocodilians, frogs, temnospondyls (extinc ...
s), forming armor
Armour (British English) or armor (American English; see spelling differences) is a covering used to protect an object, individual, or vehicle from physical injury or damage, especially direct contact weapons or projectiles during combat, or f ...
. In lepidosaurians, such as lizards and snakes, the whole skin is covered in overlapping epidermal
The epidermis is the outermost of the three layers that comprise the skin, the inner layers being the dermis and hypodermis. The epidermis layer provides a barrier to infection from environmental pathogens and regulates the amount of water relea ...
scales. Such scales were once thought to be typical of the class Reptilia as a whole, but are now known to occur only in lepidosaurians. The scales found in turtles and crocodiles are of dermal
The dermis or corium is a layer of skin between the epidermis (with which it makes up the cutis) and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided ...
, rather than epidermal, origin and are properly termed scutes. In turtles, the body is hidden inside a hard shell composed of fused scutes.
Lacking a thick dermis, reptilian leather is not as strong as mammalian leather. It is used in leather-wares for decorative purposes for shoes, belts and handbags, particularly crocodile skin.
Shedding
Reptiles shed their skin through a process called ecdysis which occurs continuously throughout their lifetime. In particular, younger reptiles tend to shed once every 5–6 weeks while adults shed 3–4 times a year. Younger reptiles shed more because of their rapid growth rate. Once full size, the frequency of shedding drastically decreases. The process of ecdysis involves forming a new layer of skin under the old one. Proteolytic
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
enzymes and lymphatic fluid
Lymph (from Latin, , meaning "water") is the fluid that flows through the lymphatic system, a system composed of lymph vessels (channels) and intervening lymph nodes whose function, like the venous system, is to return fluid from the tissues to ...
is secreted between the old and new layers of skin. Consequently, this lifts the old skin from the new one allowing shedding to occur.
Excretion
Excretion
Excretion is a process in which metabolic waste
is eliminated from an organism. In vertebrates this is primarily carried out by the lungs, kidneys, and skin. This is in contrast with secretion, where the substance may have specific tasks after ...
is performed mainly by two small kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blo ...
s. In diapsids, uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown ...
is the main nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
ous waste product; turtles, like mammals, excrete mainly urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
. Unlike the kidneys of mammals and birds, reptile kidneys are unable to produce liquid urine more concentrated than their body fluid. This is because they lack a specialized structure called a loop of Henle
In the kidney, the loop of Henle () (or Henle's loop, Henle loop, nephron loop or its Latin counterpart ''ansa nephroni'') is the portion of a nephron that leads from the proximal convoluted tubule to the distal convoluted tubule. Named after its ...
, which is present in the nephron
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure ...
s of birds and mammals. Because of this, many reptiles use the colon to aid in the reabsorption
In renal physiology, reabsorption or tubular reabsorption is the process by which the nephron removes water and solutes from the tubular fluid (pre-urine) and returns them to the circulating blood. It is called ''reabsorption'' (and not ''absorp ...
of water. Some are also able to take up water stored in the bladder
The urinary bladder, or simply bladder, is a hollow organ in humans and other vertebrates that stores urine from the kidneys before disposal by urination. In humans the bladder is a distensible organ that sits on the pelvic floor. Urine enters ...
. Excess salts are also excreted by nasal and lingual salt gland
The salt gland is an organ for excreting excess salts. It is found in the cartilaginous fishes subclass elasmobranchii (sharks, rays, and skates), seabirds, and some reptiles. Salt glands can be found in the rectum of sharks. Birds and reptiles ...
s in some reptiles.
In all reptiles the urinogenital ducts and the anus both empty into an organ called a cloaca. In some reptiles, a midventral wall in the cloaca may open into a urinary bladder, but not all. It is present in all turtles and tortoises as well as most lizards, but is lacking in the monitor lizard, the legless lizard
Legless lizard may refer to any of several groups of lizards that have independently lost limbs or reduced them to the point of being of no use in locomotion.Pough ''et al.'' 1992. Herpetology: Third Edition. Pearson Prentice Hall:Pearson Education ...
s. It is absent in the snakes, alligators, and crocodiles.
Many turtles, tortoises, and lizards have proportionally very large bladders. Charles Darwin
Charles Robert Darwin ( ; 12 February 1809 – 19 April 1882) was an English naturalist, geologist, and biologist, widely known for his contributions to evolutionary biology. His proposition that all species of life have descended ...
noted that the Galapagos tortoise had a bladder which could store up to 20% of its body weight. Such adaptations are the result of environments such as remote islands and deserts where water is very scarce. Other desert-dwelling reptiles have large bladders that can store a long-term reservoir of water for up to several months and aid in osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
.
Turtles have two or more accessory urinary bladders, located lateral to the neck of the urinary bladder and dorsal to the pubis, occupying a significant portion of their body cavity. Their bladder is also usually bilobed with a left and right section. The right section is located under the liver, which prevents large stones from remaining in that side while the left section is more likely to have calculi.
Digestion

 Most reptiles are insectivorous or carnivorous and have simple and comparatively short digestive tracts due to meat being fairly simple to break down and digest.
Most reptiles are insectivorous or carnivorous and have simple and comparatively short digestive tracts due to meat being fairly simple to break down and digest. Digestion
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intest ...
is slower than in mammals
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur o ...
, reflecting their lower resting metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
and their inability to divide and masticate their food. Their poikilotherm
A poikilotherm () is an animal whose internal temperature varies considerably. Poikilotherms have to survive and adapt to environmental stress. One of the most important stressors is temperature change, which can lead to alterations in membrane ...
metabolism has very low energy requirements, allowing large reptiles like crocodiles and large constrictors to live from a single large meal for months, digesting it slowly.[
While modern reptiles are predominantly carnivorous, during the early history of reptiles several groups produced some herbivorous megafauna: in the ]Paleozoic
The Paleozoic (or Palaeozoic) Era is the earliest of three geologic eras of the Phanerozoic Eon.
The name ''Paleozoic'' ( ;) was coined by the British geologist Adam Sedgwick in 1838
by combining the Greek words ''palaiós'' (, "old") and ' ...
, the pareiasaurs; and in the Mesozoic
The Mesozoic Era ( ), also called the Age of Reptiles, the Age of Conifers, and colloquially as the Age of the Dinosaurs is the second-to-last era of Earth's geological history, lasting from about , comprising the Triassic, Jurassic and Cretace ...
several lines of dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
s.turtle
Turtles are an order of reptiles known as Testudines, characterized by a special shell developed mainly from their ribs. Modern turtles are divided into two major groups, the Pleurodira (side necked turtles) and Cryptodira (hidden necked t ...
s are the only predominantly herbivorous reptile group, but several lines of agamas
Religion
*Āgama (Buddhism), a collection of Early Buddhist texts
*Āgama (Hinduism), scriptures of several Hindu sects
*Jain literature (Jain Āgamas), various canonical scriptures in Jainism
Other uses
* ''Agama'' (lizard), a genus of lizards ...
and iguanas
''Iguana'' (, ) is a genus of herbivorous lizards that are native to tropical areas of Mexico, Central America, South America, and the Caribbean. The genus was first described in 1768 by Austrian naturalist Josephus Nicolaus Laurenti in his ...
have evolved to live wholly or partly on plants.gastrolith
A gastrolith, also called a stomach stone or gizzard stone, is a rock held inside a gastrointestinal tract. Gastroliths in some species are retained in the muscular gizzard and used to grind food in animals lacking suitable grinding teeth. In oth ...
s) to aid in digestion: The rocks are washed around in the stomach, helping to grind up plant matter.[ Fossil gastroliths have been found associated with both ornithopods and ]sauropods
Sauropoda (), whose members are known as sauropods (; from '' sauro-'' + '' -pod'', 'lizard-footed'), is a clade of saurischian ('lizard-hipped') dinosaurs. Sauropods had very long necks, long tails, small heads (relative to the rest of their b ...
, though whether they actually functioned as a gastric mill in the latter is disputed. Salt water crocodile
The saltwater crocodile (''Crocodylus porosus'') is a crocodilian native to saltwater habitats and brackish wetlands from India's east coast across Southeast Asia and the Sundaic region to northern Australia and Micronesia. It has been listed ...
s also use gastroliths as ballast
Ballast is material that is used to provide stability to a vehicle or structure. Ballast, other than cargo, may be placed in a vehicle, often a ship or the gondola of a balloon or airship, to provide stability. A compartment within a boat, ship ...
, stabilizing them in the water or helping them to dive. A dual function as both stabilizing ballast and digestion aid has been suggested for gastroliths found in plesiosaurs.
Nerves
The reptilian nervous system contains the same basic part of the amphibian brain, but the reptile cerebrum
The cerebrum, telencephalon or endbrain is the largest part of the brain containing the cerebral cortex (of the two cerebral hemispheres), as well as several subcortical structures, including the hippocampus, basal ganglia, and olfactory bulb ...
and cerebellum are slightly larger. Most typical sense organs are well developed with certain exceptions, most notably the snake
Snakes are elongated, limbless, carnivorous reptiles of the suborder Serpentes . Like all other squamates, snakes are ectothermic, amniote vertebrates covered in overlapping scales. Many species of snakes have skulls with several more j ...
's lack of external ears (middle and inner ears are present). There are twelve pairs of cranial nerves
Cranial nerves are the nerves that emerge directly from the brain (including the brainstem), of which there are conventionally considered twelve pairs. Cranial nerves relay information between the brain and parts of the body, primarily to and f ...
. Due to their short cochlea, reptiles use electrical tuning to expand their range of audible frequencies.
Intelligence
Reptiles are generally considered less intelligent than mammals and birds.encephalization quotient
Encephalization quotient (EQ), encephalization level (EL), or just encephalization is a relative brain size measure that is defined as the ratio between observed to predicted brain mass for an animal of a given size, based on nonlinear regress ...
being about one tenth of that of mammals, though larger reptiles can show more complex brain development. Larger lizards, like the monitors, are known to exhibit complex behavior, including cooperation and cognitive abilities allowing them to optimize their foraging
Foraging is searching for wild food resources. It affects an animal's fitness because it plays an important role in an animal's ability to survive and reproduce. Foraging theory is a branch of behavioral ecology that studies the foraging behavi ...
and territoriality
In ethology, territory is the sociographical area that an animal consistently defends against conspecific competition (or, occasionally, against animals of other species) using agonistic behaviors or (less commonly) real physical aggression. ...
over time. Crocodiles have relatively larger brains and show a fairly complex social structure. The Komodo dragon
The Komodo dragon (''Varanus komodoensis''), also known as the Komodo monitor, is a member of the monitor lizard family Varanidae that is endemic to the Indonesian islands of Komodo, Rinca, Flores, and Gili Motang. It is the largest extant ...
is even known to engage in play,wood turtle
The wood turtle (''Glyptemys insculpta'') is a species of turtle endemic to North America. It is in the genus ''Glyptemys'', a genus which contains only one other species of turtle: the bog turtle (''Glyptemys muhlenbergii'' ). The wood turtle ...
s were better than white rats at learning to navigate mazes. Another study found that giant tortoises are capable of learning through operant conditioning, visual discrimination and retained learned behaviors with long-term memory. Sea turtles have been regarded as having simple brains, but their flippers are used for a variety of foraging tasks (holding, bracing, corralling) in common with marine mammals.
Vision
Most reptiles are diurnal animals. The vision is typically adapted to daylight conditions, with color vision and more advanced visual depth perception than in amphibians and most mammals.
Reptiles usually have excellent vision, allowing them to detect shapes and motions at long distances. They often have only a few Rod cells and have poor vision in low-light conditions. At the same time they have cells called "double cone
A cone is a three-dimensional geometric shape that tapers smoothly from a flat base (frequently, though not necessarily, circular) to a point called the apex or vertex.
A cone is formed by a set of line segments, half-lines, or lines con ...
s" which give them sharp color vision and enable them to see ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
wavelengths.parietal eye
A parietal eye, also known as a third eye or pineal eye, is a part of the epithalamus present in some vertebrates. The eye is located at the top of the head, is photoreceptive and is associated with the pineal gland, regulating circadian rhyth ...
, which are also called third eye
The third eye (also called the mind's eye or inner eye) is a mystical invisible eye, usually depicted as located on the forehead, which provides perception beyond ordinary sight. In Hinduism, the third eye refers to the ajna (or brow) chakra. In ...
, pineal eye or pineal gland. This "eye" does not work the same way as a normal eye does as it has only a rudimentary retina and lens and thus, cannot form images. It is, however, sensitive to changes in light and dark and can detect movement.[
Some snakes have extra sets of visual organs (in the loosest sense of the word) in the form of pits sensitive to ]infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
radiation (heat). Such heat-sensitive pits are particularly well developed in the pit vipers, but are also found in boas and pythons
The Pythonidae, commonly known as pythons, are a family of nonvenomous snakes found in Africa, Asia, and Australia. Among its members are some of the largest snakes in the world. Ten genera and 42 species are currently recognized.
Distribution ...
. These pits allow the snakes to sense the body heat of birds and mammals, enabling pit vipers to hunt rodents in the dark.
Most reptiles, as well as birds, possess a nictitating membrane, a translucent third eyelid which is drawn over the eye from the inner corner. In crocodilians , it protects its eyeball surface while allowing a degree of vision underwater. However, many squamates, geckos and snakes in particular, lack eyelids, which are replaced by a transparent scale. This is called the brille, spectacle, or eyecap. The brille is usually not visible, except for when the snake molts, and it protects the eyes from dust and dirt.
Reproduction


 Reptiles generally reproduce sexually, though some are capable of asexual reproduction. All reproductive activity occurs through the cloaca, the single exit/entrance at the base of the tail where waste is also eliminated. Most reptiles have copulatory organs, which are usually retracted or inverted and stored inside the body. In turtles and crocodilians, the male has a single median
Reptiles generally reproduce sexually, though some are capable of asexual reproduction. All reproductive activity occurs through the cloaca, the single exit/entrance at the base of the tail where waste is also eliminated. Most reptiles have copulatory organs, which are usually retracted or inverted and stored inside the body. In turtles and crocodilians, the male has a single median penis
A penis (plural ''penises'' or ''penes'' () is the primary sexual organ that male animals use to inseminate females (or hermaphrodites) during copulation. Such organs occur in many animals, both vertebrate and invertebrate, but males d ...
, while squamates, including snakes and lizards, possess a pair of hemipenes, only one of which is typically used in each session. Tuatara, however, lack copulatory organs, and so the male and female simply press their cloacas together as the male discharges sperm.
Most reptiles lay amniotic eggs covered with leathery or calcareous shells. An amnion, chorion
The chorion is the outermost fetal membrane around the embryo in mammals, birds and reptiles (amniotes). It develops from an outer fold on the surface of the yolk sac, which lies outside the zona pellucida (in mammals), known as the vitell ...
, and allantois are present during embryonic life. The eggshell (1) protects the crocodile embryo (11) and keeps it from drying out, but it is flexible to allow gas exchange. The chorion (6) aids in gas exchange between the inside and outside of the egg. It allows carbon dioxide to exit the egg and oxygen gas to enter the egg. The albumin (9) further protects the embryo and serves as a reservoir for water and protein. The allantois (8) is a sac that collects the metabolic waste produced by the embryo. The amniotic sac (10) contains amniotic fluid (12) which protects and cushions the embryo. The amnion (5) aids in osmoregulation and serves as a saltwater reservoir. The yolk sac (2) surrounding the yolk (3) contains protein and fat rich nutrients that are absorbed by the embryo via vessels (4) that allow the embryo to grow and metabolize. The air space (7) provides the embryo with oxygen while it is hatching. This ensures that the embryo will not suffocate while it is hatching. There are no larva
A larva (; plural larvae ) is a distinct juvenile form many animals undergo before metamorphosis into adults. Animals with indirect development such as insects, amphibians, or cnidarians typically have a larval phase of their life cycle.
...
l stages of development. Viviparity
Among animals, viviparity is development of the embryo inside the body of the parent. This is opposed to oviparity which is a reproductive mode in which females lay developing eggs that complete their development and hatch externally from the ...
and ovoviviparity have evolved in many extinct clades of reptiles and in squamates. In the latter group, many species, including all boas and most vipers, use this mode of reproduction. The degree of viviparity varies; some species simply retain the eggs until just before hatching, others provide maternal nourishment to supplement the yolk, and yet others lack any yolk and provide all nutrients via a structure similar to the mammalian placenta
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate mate ...
. The earliest documented case of viviparity in reptiles is the Early Permian
The Permian ( ) is a geologic period and System (stratigraphy), stratigraphic system which spans 47 million years from the end of the Carboniferous Period million years ago (Mya), to the beginning of the Triassic Period 251.9 Mya. It is the last ...
mesosaur
Mesosaurs ("middle lizards") were a group of small aquatic reptiles that lived during the early Permian period, roughly 299 to 270 million years ago. Mesosaurs were the first known aquatic reptiles, having apparently returned to an aquatic life ...
s,mosasaur
Mosasaurs (from Latin ''Mosa'' meaning the 'Meuse', and Greek ' meaning 'lizard') comprise a group of extinct, large marine reptiles from the Late Cretaceous. Their first fossil remains were discovered in a limestone quarry at Maastricht on ...
s, ichthyosaurs, and Sauropterygia
Sauropterygia ("lizard flippers") is an extinct taxon of diverse, aquatic reptiles that developed from terrestrial ancestors soon after the end-Permian extinction and flourished during the Triassic before all except for the Plesiosauria became ...
, a group that include pachypleurosaur
left, 220px, '' Pachypleurosaurus''
Pachypleurosauria is an extinct clade of primitive sauropterygian reptiles that vaguely resembled aquatic lizards, and were limited to the Triassic period. They were elongate animals, ranging in size from , w ...
s and Plesiosauria
The Plesiosauria (; Greek: πλησίος, ''plesios'', meaning "near to" and ''sauros'', meaning "lizard") or plesiosaurs are an order or clade of extinct Mesozoic marine reptiles, belonging to the Sauropterygia.
Plesiosaurs first appeared ...
.squamates
Squamata (, Latin ''squamatus'', 'scaly, having scales') is the largest order of reptiles, comprising lizards, snakes, and amphisbaenians (worm lizards), which are collectively known as squamates or scaled reptiles. With over 10,900 species, it ...
in six families of lizards and one snake. In some species of squamates, a population of females is able to produce a unisexual diploid clone of the mother. This form of asexual reproduction, called parthenogenesis
Parthenogenesis (; from the Greek grc, παρθένος, translit=parthénos, lit=virgin, label=none + grc, γένεσις, translit=génesis, lit=creation, label=none) is a natural form of asexual reproduction in which growth and developmen ...
, occurs in several species of gecko, and is particularly widespread in the teiids (especially ''Aspidocelis'') and lacertids (''Lacerta
Lacerta is one of the 88 modern constellations defined by the International Astronomical Union. Its name is Latin for lizard. A small, faint constellation, it was defined in 1687 by the astronomer Johannes Hevelius. Its brightest stars form a "W" ...
''). In captivity, Komodo dragon
The Komodo dragon (''Varanus komodoensis''), also known as the Komodo monitor, is a member of the monitor lizard family Varanidae that is endemic to the Indonesian islands of Komodo, Rinca, Flores, and Gili Motang. It is the largest extant ...
s (Varanidae) have reproduced by parthenogenesis
Parthenogenesis (; from the Greek grc, παρθένος, translit=parthénos, lit=virgin, label=none + grc, γένεσις, translit=génesis, lit=creation, label=none) is a natural form of asexual reproduction in which growth and developmen ...
.
Parthenogenetic species are suspected to occur among chameleons, agamids, xantusiids, and typhlopids.
Some reptiles exhibit temperature-dependent sex determination Temperature-dependent sex determination (TSD) is a type of environmental sex determination in which the temperatures experienced during embryonic/larval development determine the sex of the offspring. It is only observed in reptiles and teleost fish ...
(TDSD), in which the incubation temperature determines whether a particular egg hatches as male or female. TDSD is most common in turtles and crocodiles, but also occurs in lizards and tuatara. To date, there has been no confirmation of whether TDSD occurs in snakes.
Defense mechanisms
Many small reptiles, such as snakes and lizards, that live on the ground or in the water are vulnerable to being preyed on by all kinds of carnivorous animals. Thus, avoidance is the most common form of defense in reptiles. At the first sign of danger, most snakes and lizards crawl away into the undergrowth, and turtles and crocodiles will plunge into water and sink out of sight.
Camouflage and warning
 Reptiles tend to avoid confrontation through
Reptiles tend to avoid confrontation through camouflage
Camouflage is the use of any combination of materials, coloration, or illumination for concealment, either by making animals or objects hard to see, or by disguising them as something else. Examples include the leopard's spotted coat, the b ...
. Two major groups of reptile predators are birds and other reptiles, both of which have well developed color vision. Thus the skins of many reptiles have cryptic coloration of plain or mottled gray, green, and brown to allow them to blend into the background of their natural environment. Aided by the reptiles' capacity for remaining motionless for long periods, the camouflage of many snakes is so effective that people or domestic animals are most typically bitten because they accidentally step on them.
When camouflage fails to protect them, blue-tongued skink
Blue-tongued skinks comprise the Australasian genus ''Tiliqua'', which contains some of the largest members of the skink family (Scincidae). They are commonly called blue-tongued lizards or simply blue-tongues or blueys in Australia. As suggeste ...
s will try to ward off attackers by displaying their blue tongues, and the frill-necked lizard
The frilled lizard (''Chlamydosaurus kingii''), also known as the frill-necked lizard or frilled dragon, is a species of lizard in the family Agamidae. It is native to northern Australia and southern New Guinea. This species is the only member o ...
will display its brightly colored frill. These same displays are used in territorial disputes and during courtship.Rattlesnakes
Rattlesnakes are venomous snakes that form the genera '' Crotalus'' and '' Sistrurus'' of the subfamily Crotalinae (the pit vipers). All rattlesnakes are vipers. Rattlesnakes are predators that live in a wide array of habitats, hunting small an ...
rapidly vibrate the tip of the tail, which is composed of a series of nested, hollow beads to ward off approaching danger.
In contrast to the normal drab coloration of most reptiles, the lizards of the genus ''Heloderma'' (the Gila monster
The Gila monster (''Heloderma suspectum'', ) is a species of venomous lizard native to the Southwestern United States and the northwestern Mexican state of Sonora. It is a heavy, typically slow-moving reptile, up to long, and it is the only ve ...
and the beaded lizard) and many of the coral snake
Coral snakes are a large group of elapid snakes that can be divided into two distinct groups, the Old World coral snakes and New World coral snakes. There are 16 species of Old World coral snakes, in three genera (''Calliophis'', '' Hemibungar ...
s have high-contrast warning coloration, warning potential predators they are venomous. A number of non-venomous North American snake species have colorful markings similar to those of the coral snake, an oft cited example of Batesian mimicry.
Alternative defense in snakes
Camouflage does not always fool a predator. When caught out, snake species adopt different defensive tactics and use a complicated set of behaviors when attacked. Some species, like cobras or hognose snakes, first elevate their head and spread out the skin of their neck in an effort to look large and threatening. Failure of this strategy may lead to other measures practiced particularly by cobras, vipers, and closely related species, which use venom
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved ''venom apparatus'', such as fangs or a st ...
to attack. The venom is modified saliva, delivered through fangs from a venom gland. Some non-venomous snakes, such as American hognose snakes
Hognose snake is a common name for several unrelated species of snake with upturned snouts, classified in 2 colubrid snake genera and 1 pseudoxyrhophiid snake genus.
They include the following genera:
*'' Heterodon'', which occur mainly in ...
or European grass snake
The grass snake (''Natrix natrix''), sometimes called the ringed snake or water snake, is a Eurasian non-venomous colubrid snake. It is often found near water and feeds almost exclusively on amphibians.
Subspecies
Many subspecies are recognized ...
, play dead when in danger; some, including the grass snake, exude a foul-smelling liquid to deter attackers.
Defense in crocodilians
When a crocodilian is concerned about its safety, it will gape to expose the teeth and tongue. If this does not work, the crocodilian gets a little more agitated and typically begins to make hissing sounds. After this, the crocodilian will start to change its posture dramatically to make itself look more intimidating. The body is inflated to increase apparent size. If absolutely necessary it may decide to attack an enemy.
 Some species try to bite immediately. Some will use their heads as sledgehammers and literally smash an opponent, some will rush or swim toward the threat from a distance, even chasing the opponent onto land or galloping after it. The main weapon in all crocodiles is the bite, which can generate very high bite force. Many species also possess canine-like teeth. These are used primarily for seizing prey, but are also used in fighting and display.
Some species try to bite immediately. Some will use their heads as sledgehammers and literally smash an opponent, some will rush or swim toward the threat from a distance, even chasing the opponent onto land or galloping after it. The main weapon in all crocodiles is the bite, which can generate very high bite force. Many species also possess canine-like teeth. These are used primarily for seizing prey, but are also used in fighting and display.
Shedding and regenerating tails
Geckos
Geckos are small, mostly carnivorous lizards that have a wide distribution, found on every continent except Antarctica. Belonging to the infraorder Gekkota, geckos are found in warm climates throughout the world. They range from .
Geckos ar ...
, skinks
Skinks are lizards belonging to the family Scincidae, a family in the infraorder Scincomorpha. With more than 1,500 described species across 100 different taxonomic genera, the family Scincidae is one of the most diverse families of lizards. Sk ...
, and some other lizards that are captured by the tail will shed part of the tail structure through a process called autotomy and thus be able to flee. The detached tail will continue to thrash, creating a deceptive sense of continued struggle and distracting the predator's attention from the fleeing prey animal. The detached tails of leopard gecko
The leopard gecko or common leopard gecko (''Eublepharis macularius'') is a ground-dwelling lizard native to the rocky dry grassland and desert regions of Afghanistan, Iran, Pakistan, India, and Nepal. The leopard gecko has become a popular pet, ...
s can wiggle for up to 20 minutes. The tail grows back in most species, but some, like crested geckos, lose their tails for the rest of their lives. In many species the tails are of a separate and dramatically more intense color than the rest of the body so as to encourage potential predators to strike for the tail first. In the shingleback skink and some species of geckos, the tail is short and broad and resembles the head, so that the predators may attack it rather than the more vulnerable front part.
Relations with humans
In cultures and religions
 Dinosaurs have been widely depicted in culture since the English palaeontologist Richard Owen coined the name ''
Dinosaurs have been widely depicted in culture since the English palaeontologist Richard Owen coined the name ''dinosaur
Dinosaurs are a diverse group of reptiles of the clade Dinosauria. They first appeared during the Triassic period, between 243 and 233.23 million years ago (mya), although the exact origin and timing of the evolution of dinosaurs is t ...
'' in 1842. As soon as 1854, the Crystal Palace Dinosaurs were on display to the public in south London.[Torrens, Hugh. "Politics and Paleontology". ''The Complete Dinosaur,'' 175–190.]Charles Dickens
Charles John Huffam Dickens (; 7 February 1812 – 9 June 1870) was an English writer and social critic. He created some of the world's best-known fictional characters and is regarded by many as the greatest novelist of the Victorian e ...
placed a ''Megalosaurus
''Megalosaurus'' (meaning "great lizard", from Greek , ', meaning 'big', 'tall' or 'great' and , ', meaning 'lizard') is an extinct genus of large carnivorous theropod dinosaurs of the Middle Jurassic period (Bathonian stage, 166 million years ...
'' in the first chapter of his novel ''Bleak House
''Bleak House'' is a novel by Charles Dickens, first published as a 20-episode serial between March 1852 and September 1853. The novel has many characters and several sub-plots, and is told partly by the novel's heroine, Esther Summerson, and ...
'' in 1852. The dinosaurs featured in books, films, television programs, artwork, and other media have been used for both education and entertainment. The depictions range from the realistic, as in the television documentaries
A documentary film or documentary is a non-fictional motion-picture intended to "document reality, primarily for the purposes of instruction, education or maintaining a historical record". Bill Nichols has characterized the documentary in term ...
of the 1990s and first decade of the 21st century, or the fantastic, as in the monster movie
A monster movie, monster film, creature feature or giant monster film is a film that focuses on one or more characters struggling to survive attacks by one or more antagonistic monsters, often abnormally large ones. The film may also fall under ...
s of the 1950s and 1960s.pharaoh
Pharaoh (, ; Egyptian: '' pr ꜥꜣ''; cop, , Pǝrro; Biblical Hebrew: ''Parʿō'') is the vernacular term often used by modern authors for the kings of ancient Egypt who ruled as monarchs from the First Dynasty (c. 3150 BC) until the ...
. It was worshipped as one of the gods and was also used for sinister purposes: murder of an adversary and ritual suicide ( Cleopatra). In Greek mythology
A major branch of classical mythology, Greek mythology is the body of myths originally told by the ancient Greeks, and a genre of Ancient Greek folklore. These stories concern the origin and nature of the world, the lives and activities ...
snakes are associated with deadly antagonists, as a chthonic symbol, roughly translated as ''earthbound''. The nine-headed Lernaean Hydra
The Lernaean Hydra or Hydra of Lerna ( grc-gre, Λερναῖα Ὕδρα, ''Lernaîa Hýdra''), more often known simply as the Hydra, is a snake, serpentine water monster in Greek mythology, Greek and Roman mythology. Its lair was the lake of Le ...
that Hercules
Hercules (, ) is the Roman equivalent of the Greek divine hero Heracles, son of Jupiter and the mortal Alcmena. In classical mythology, Hercules is famous for his strength and for his numerous far-ranging adventures.
The Romans adapted the ...
defeated and the three Gorgon
A Gorgon ( /ˈɡɔːrɡən/; plural: Gorgons, Ancient Greek: Γοργών/Γοργώ ''Gorgṓn/Gorgṓ'') is a creature in Greek mythology. Gorgons occur in the earliest examples of Greek literature. While descriptions of Gorgons vary, the te ...
sisters are children of Gaia, the earth. Medusa
In Greek mythology, Medusa (; Ancient Greek: Μέδουσα "guardian, protectress"), also called Gorgo, was one of the three monstrous Gorgons, generally described as winged human females with living venomous snakes in place of hair. Those ...
was one of the three Gorgon sisters who Perseus defeated. Medusa is described as a hideous mortal, with snakes instead of hair and the power to turn men to stone with her gaze. After killing her, Perseus gave her head to Athena
Athena or Athene, often given the epithet Pallas, is an ancient Greek religion, ancient Greek goddess associated with wisdom, warfare, and handicraft who was later syncretism, syncretized with the Roman goddess Minerva. Athena was regarded ...
who fixed it to her shield called the Aegis
The aegis ( ; grc, αἰγίς ''aigís''), as stated in the ''Iliad'', is a device carried by Athena and Zeus, variously interpreted as an animal skin or a shield and sometimes featuring the head of a Gorgon. There may be a connection with a d ...
. The Titans
In Greek mythology, the Titans ( grc, οἱ Τῑτᾶνες, ''hoi Tītânes'', , ''ho Tītân'') were the pre-Olympian gods. According to the ''Theogony'' of Hesiod, they were the twelve children of the primordial parents Uranus (Sky) and Gai ...
are depicted in art with their legs replaced by bodies of snakes for the same reason: They are children of Gaia, so they are bound to the earth.Shiva
Shiva (; sa, शिव, lit=The Auspicious One, Śiva ), also known as Mahadeva (; ɐɦaːd̪eːʋɐ, or Hara, is one of the principal deities of Hinduism. He is the Supreme Being in Shaivism, one of the major traditions within Hindu ...
, while Vishnu
Vishnu ( ; , ), also known as Narayana and Hari, is one of the principal deities of Hinduism. He is the supreme being within Vaishnavism, one of the major traditions within contemporary Hinduism.
Vishnu is known as "The Preserver" withi ...
is depicted often as sleeping on a seven-headed snake or within the coils of a serpent. There are temples in India solely for cobras sometimes called ''Nagraj'' (King of Snakes), and it is believed that snakes are symbols of fertility. In the annual Hindu festival of Nag Panchami, snakes are venerated and prayed to.Mesoamerica
Mesoamerica is a historical region and cultural area in southern North America and most of Central America. It extends from approximately central Mexico through Belize, Guatemala, El Salvador, Honduras, Nicaragua, and northern Costa Rica ...
. "In states of ecstasy, lords dance a serpent dance; great descending snakes adorn and support buildings from Chichen Itza to Tenochtitlan
, ; es, Tenochtitlan also known as Mexico-Tenochtitlan, ; es, México-Tenochtitlan was a large Mexican in what is now the historic center of Mexico City. The exact date of the founding of the city is unclear. The date 13 March 1325 was ...
, and the Nahuatl word ''coatl'' meaning serpent or twin, forms part of primary deities such as Mixcoatl
Mixcoatl ( nah, Mixcōhuātl}, from mixtli "cloud" and cōātl "serpent"), or Camaxtle or Camaxtli, was the god of the hunt and identified with the Milky Way, the stars, and the heavens in several Mesoamerican cultures. He was the patron deity ...
, Quetzalcoatl, and Coatlicue." In Christianity and Judaism, a serpent appears in Genesis to tempt Adam and Eve
Adam and Eve, according to the creation myth of the Abrahamic religions, were the first man and woman. They are central to the belief that humanity is in essence a single family, with everyone descended from a single pair of original ancestors. ...
with the forbidden fruit
Forbidden fruit is a name given to the fruit growing in the Garden of Eden which God commands mankind not to eat. In the biblical story, Adam and Eve eat the fruit from the tree of the knowledge of good and evil and are exiled from Eden.
As a ...
from the Tree of Knowledge of Good and Evil
In Judaism and Christianity, the tree of the knowledge of good and evil ( he, עֵץ הַדַּעַת טוֹב וָרָע, ʿêṣ had-daʿaṯ ṭōḇ wā-rāʿ, label=Tiberian Hebrew, ) is one of two specific trees in the story of the Garden ...
.
The turtle has a prominent position as a symbol of steadfastness and tranquility in religion, mythology, and folklore from around the world.[Plotkin, Pamela, T., 2007, ''Biology and Conservation of Ridley Sea Turtles'', Johns Hopkins University, .] A tortoise's longevity is suggested by its long lifespan and its shell, which was thought to protect it from any foe.[Ball, Catherine, 2004, ''Animal Motifs in Asian Art'', Courier Dover Publications, .] In the cosmological myth
A creation myth (or cosmogonic myth) is a symbolic narrative of how the world began and how people first came to inhabit it., "Creation myths are symbolic stories describing how the universe and its inhabitants came to be. Creation myths develop ...
s of several cultures a ''World Turtle
The World Turtle, also called the Cosmic Turtle or the World-bearing Turtle, is a mytheme of a giant turtle (or tortoise) supporting or containing the world. It occurs in Hindu mythology, Chinese mythology, and the mythologies of the indigenous p ...
'' carries the world upon its back or supports the heavens.[Stookey, Lorena Laura, 2004, ''Thematic Guide to World Mythology'', Greenwood Press, .]
Medicine
 Deaths from
Deaths from snakebite
A snakebite is an injury caused by the bite of a snake, especially a venomous snake. A common sign of a bite from a venomous snake is the presence of two puncture wounds from the animal's fangs. Sometimes venom injection from the bite may occu ...
s are uncommon in many parts of the world, but are still counted in tens of thousands per year in India.antivenom
Antivenom, also known as antivenin, venom antiserum, and antivenom immunoglobulin, is a specific treatment for envenomation. It is composed of antibodies and used to treat certain venomous bites and stings. Antivenoms are recommended only if th ...
made from the venom of the snake. To produce antivenom, a mixture of the venoms of different species of snake is injected into the body of a horse in ever-increasing dosages until the horse is immunized. Blood is then extracted; the serum is separated, purified and freeze-dried. The cytotoxic effect of snake venom is being researched as a potential treatment for cancers.
Lizards such as the Gila monster produce toxins with medical applications. Gila toxin reduces plasma glucose; the substance is now synthesised for use in the anti-diabetes
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ...
drug exenatide
Exenatide, sold under the brand name Byetta and Bydureon among others, is a medication used to treat diabetes mellitus type 2. It is used together with diet, exercise, and potentially other antidiabetic medication. It is a treatment option after ...
(Byetta).
Commercial farming
Crocodiles are protected in many parts of the world, and are farmed commercially. Their hides are tanned and used to make leather goods such as shoes and handbag
A handbag, commonly known as a purse or pocketbook in North American English, is a handled medium-to-large bag used to carry personal items.
Purse, handbag or pouch
The term "purse" originally referred to a small bag for holding coins. In man ...
s; crocodile meat is also considered a delicacy. The most commonly farmed species are the saltwater and Nile crocodiles. Farming has resulted in an increase in the saltwater crocodile population in Australia, as eggs are usually harvested from the wild, so landowners have an incentive to conserve their habitat. Crocodile leather is made into wallets, briefcases, purses, handbags, belts, hats, and shoes. Crocodile oil has been used for various purposes.
Snakes are also farmed, primarily in East
East or Orient is one of the four cardinal directions or points of the compass. It is the opposite direction from west and is the direction from which the Sun rises on the Earth.
Etymology
As in other languages, the word is formed from the fac ...
and Southeast Asia
Southeast Asia, also spelled South East Asia and South-East Asia, and also known as Southeastern Asia, South-eastern Asia or SEA, is the geographical south-eastern region of Asia, consisting of the regions that are situated south of mainlan ...
, and their production has become more intensive in the last decade. Snake farm
A snake farm is a facility that houses and breeds a wide variety of snakes, often for the purpose of research and the collection of venom for the creation of antivenom. Many snake farms are primarily tourist attractions. Notable snake farms exist ...
ing has been troubling for conservation in the past as it can lead to overexploitation of wild snakes and their natural prey to supply the farms. However, farming snakes can limit the hunting of wild snakes, while reducing the slaughter of higher-order vertebrates like cows. The energy efficiency of snakes is higher than expected for carnivores, due to their ectothermy and low metabolism. Waste protein from the poultry and pig industries is used as feed in snake farms. Snake farms produce meat, snake skin, and antivenom.
Turtle farming is another known but controversial practice. Turtles have been farmed for a variety of reasons, ranging from food to traditional medicine, the pet trade, and scientific conservation. Demand for turtle meat and medicinal products is one of the main threats to turtle conservation in Asia. Though commercial breeding would seem to insulate wild populations, it can stoke the demand for them and increase wild captures.
Reptiles in captivity
In the Western world, some snakes (especially relatively docile species such as the ball python
The ball python (''Python regius''), also called the royal python, is a python species native to West and Central Africa, where it lives in grasslands, shrublands and open forests. This nonvenomous constrictor is the smallest of the African p ...
and corn snake) are sometimes kept as pets. Numerous species of lizard are kept as pet
A pet, or companion animal, is an animal kept primarily for a person's company or entertainment rather than as a working animal, livestock, or a laboratory animal. Popular pets are often considered to have attractive appearances, intelligence ...
s, including bearded dragons
''Pogona'' is a genus of reptiles containing six lizard species which are often known by the common name bearded dragons. The name "bearded dragon" refers to the underside of the throat (or "beard") of the lizard, which can turn black and gain we ...
,iguana
''Iguana'' (, ) is a genus of herbivorous lizards that are native to tropical areas of Mexico, Central America, South America, and the Caribbean. The genus was first described in 1768 by Austrian naturalist Josephus Nicolaus Laurenti in his ...
s, anoles, and geckos (such as the popular leopard gecko
The leopard gecko or common leopard gecko (''Eublepharis macularius'') is a ground-dwelling lizard native to the rocky dry grassland and desert regions of Afghanistan, Iran, Pakistan, India, and Nepal. The leopard gecko has become a popular pet, ...
and the crested gecko).herpetarium
A herpetarium is a zoological exhibition space for reptiles and amphibians, most commonly a dedicated area of a larger zoo. A herpetarium which specializes in snakes is an ophidiarium or serpentarium, which are more common as stand-alone entiti ...
is a zoological exhibition space for reptiles and amphibians.
See also
* List of reptiles
* Lists of reptiles by region
The following are the regional reptiles lists by continent.
Continent
Africa
*Canary Islands
*Democratic Republic of the Congo
*Egypt
*Ghana
*Lesotho
*Madagascar
*Morocco
Asia
*South Asia
*Korean Peninsula
*Afghanistan
*China
*India
**Kaziran ...
*
Further reading
*
*
*
*
Notes
References
External links
*
*
*
Reptile Phylogeny
Reptile images
Sri Lanka Wild Life Information Database
''Biology of the Reptilia''
is an online copy of the full text of a 22 volume 13,000 page summary of the state of research of reptiles.
{{Authority control
Extant Pennsylvanian first appearances
Paraphyletic groups
Articles containing video clips
 In the 13th century the category of ''reptile'' was recognized in Europe as consisting of a miscellany of egg-laying creatures, including "snakes, various fantastic monsters, lizards, assorted amphibians, and worms", as recorded by
In the 13th century the category of ''reptile'' was recognized in Europe as consisting of a miscellany of egg-laying creatures, including "snakes, various fantastic monsters, lizards, assorted amphibians, and worms", as recorded by  It was not until the beginning of the 19th century that it became clear that reptiles and amphibians are, in fact, quite different animals, and
It was not until the beginning of the 19th century that it became clear that reptiles and amphibians are, in fact, quite different animals, and  The synapsid/sauropsid division supplemented another approach, one that split the reptiles into four subclasses based on the number and position of
The synapsid/sauropsid division supplemented another approach, one that split the reptiles into four subclasses based on the number and position of  The composition of Euryapsida was uncertain.
The composition of Euryapsida was uncertain.  When Sauropsida was used, it often had the same content or even the same definition as Reptilia. In 1988, Jacques Gauthier proposed a
When Sauropsida was used, it often had the same content or even the same definition as Reptilia. In 1988, Jacques Gauthier proposed a 
 The origin of the reptiles lies about 310–320 million years ago, in the steaming swamps of the late Carboniferous period, when the first reptiles evolved from advanced reptiliomorphs.
The oldest known animal that may have been an amniote is ''
The origin of the reptiles lies about 310–320 million years ago, in the steaming swamps of the late Carboniferous period, when the first reptiles evolved from advanced reptiliomorphs.
The oldest known animal that may have been an amniote is '' It was traditionally assumed that the first reptiles retained an
It was traditionally assumed that the first reptiles retained an 
 The close of the
The close of the  All lepidosaurs and
All lepidosaurs and  For example,
For example,  Modern non-avian reptiles exhibit some form of cold-bloodedness (i.e. some mix of
Modern non-avian reptiles exhibit some form of cold-bloodedness (i.e. some mix of  How turtles and tortoises breathe has been the subject of much study. To date, only a few species have been studied thoroughly enough to get an idea of how those turtles breathe. The varied results indicate that turtles and tortoises have found a variety of solutions to this problem.
The difficulty is that most turtle shells are rigid and do not allow for the type of expansion and contraction that other amniotes use to ventilate their lungs. Some turtles, such as the Indian flapshell ('' Lissemys punctata''), have a sheet of muscle that envelops the lungs. When it contracts, the turtle can exhale. When at rest, the turtle can retract the limbs into the body cavity and force air out of the lungs. When the turtle protracts its limbs, the pressure inside the lungs is reduced, and the turtle can suck air in. Turtle lungs are attached to the inside of the top of the shell (carapace), with the bottom of the lungs attached (via connective tissue) to the rest of the viscera. By using a series of special muscles (roughly equivalent to a diaphragm), turtles are capable of pushing their viscera up and down, resulting in effective respiration, since many of these muscles have attachment points in conjunction with their forelimbs (indeed, many of the muscles expand into the limb pockets during contraction).
Breathing during locomotion has been studied in three species, and they show different patterns. Adult female green sea turtles do not breathe as they crutch along their nesting beaches. They hold their breath during terrestrial locomotion and breathe in bouts as they rest. North American box turtles breathe continuously during locomotion, and the ventilation cycle is not coordinated with the limb movements. This is because they use their abdominal muscles to breathe during locomotion. The last species to have been studied is the red-eared slider, which also breathes during locomotion, but takes smaller breaths during locomotion than during small pauses between locomotor bouts, indicating that there may be mechanical interference between the limb movements and the breathing apparatus. Box turtles have also been observed to breathe while completely sealed up inside their shells.
How turtles and tortoises breathe has been the subject of much study. To date, only a few species have been studied thoroughly enough to get an idea of how those turtles breathe. The varied results indicate that turtles and tortoises have found a variety of solutions to this problem.
The difficulty is that most turtle shells are rigid and do not allow for the type of expansion and contraction that other amniotes use to ventilate their lungs. Some turtles, such as the Indian flapshell ('' Lissemys punctata''), have a sheet of muscle that envelops the lungs. When it contracts, the turtle can exhale. When at rest, the turtle can retract the limbs into the body cavity and force air out of the lungs. When the turtle protracts its limbs, the pressure inside the lungs is reduced, and the turtle can suck air in. Turtle lungs are attached to the inside of the top of the shell (carapace), with the bottom of the lungs attached (via connective tissue) to the rest of the viscera. By using a series of special muscles (roughly equivalent to a diaphragm), turtles are capable of pushing their viscera up and down, resulting in effective respiration, since many of these muscles have attachment points in conjunction with their forelimbs (indeed, many of the muscles expand into the limb pockets during contraction).
Breathing during locomotion has been studied in three species, and they show different patterns. Adult female green sea turtles do not breathe as they crutch along their nesting beaches. They hold their breath during terrestrial locomotion and breathe in bouts as they rest. North American box turtles breathe continuously during locomotion, and the ventilation cycle is not coordinated with the limb movements. This is because they use their abdominal muscles to breathe during locomotion. The last species to have been studied is the red-eared slider, which also breathes during locomotion, but takes smaller breaths during locomotion than during small pauses between locomotor bouts, indicating that there may be mechanical interference between the limb movements and the breathing apparatus. Box turtles have also been observed to breathe while completely sealed up inside their shells.
 Reptilian skin is covered in a horny epidermis, making it watertight and enabling reptiles to live on dry land, in contrast to amphibians. Compared to mammalian skin, that of reptiles is rather thin and lacks the thick
Reptilian skin is covered in a horny epidermis, making it watertight and enabling reptiles to live on dry land, in contrast to amphibians. Compared to mammalian skin, that of reptiles is rather thin and lacks the thick 
 Most reptiles are insectivorous or carnivorous and have simple and comparatively short digestive tracts due to meat being fairly simple to break down and digest.
Most reptiles are insectivorous or carnivorous and have simple and comparatively short digestive tracts due to meat being fairly simple to break down and digest. 
 Reptiles generally reproduce sexually, though some are capable of asexual reproduction. All reproductive activity occurs through the cloaca, the single exit/entrance at the base of the tail where waste is also eliminated. Most reptiles have copulatory organs, which are usually retracted or inverted and stored inside the body. In turtles and crocodilians, the male has a single median
Reptiles generally reproduce sexually, though some are capable of asexual reproduction. All reproductive activity occurs through the cloaca, the single exit/entrance at the base of the tail where waste is also eliminated. Most reptiles have copulatory organs, which are usually retracted or inverted and stored inside the body. In turtles and crocodilians, the male has a single median  Reptiles tend to avoid confrontation through
Reptiles tend to avoid confrontation through  Some species try to bite immediately. Some will use their heads as sledgehammers and literally smash an opponent, some will rush or swim toward the threat from a distance, even chasing the opponent onto land or galloping after it. The main weapon in all crocodiles is the bite, which can generate very high bite force. Many species also possess canine-like teeth. These are used primarily for seizing prey, but are also used in fighting and display.
Some species try to bite immediately. Some will use their heads as sledgehammers and literally smash an opponent, some will rush or swim toward the threat from a distance, even chasing the opponent onto land or galloping after it. The main weapon in all crocodiles is the bite, which can generate very high bite force. Many species also possess canine-like teeth. These are used primarily for seizing prey, but are also used in fighting and display.
 Dinosaurs have been widely depicted in culture since the English palaeontologist Richard Owen coined the name ''
Dinosaurs have been widely depicted in culture since the English palaeontologist Richard Owen coined the name ''