Porphyrin ring on:
[Wikipedia]
[Google]
[Amazon]
 Porphyrins ( ) are a group of
Porphyrins ( ) are a group of
File:PPIXtransH.png, Derivatives of

 Porphyrins are often used to construct structures in
Porphyrins are often used to construct structures in
File:H2TPP.png, Lewis structure for ''meso''-tetraphenylporphyrin
File:Meso-tetraphenylporphyrin UV-vis.JPG, UV–vis readout for ''meso''-tetraphenylporphyrin
File:Porfirina activada con la luz.svg, Light-activated porphyrin. Monatomic oxygen. Cellular aging
Journal of Porphyrins and Phthalocyanines
Porphynet – an informative site about porphyrins and related structures
{{Authority control Biomolecules Metabolism Photosynthetic pigments Chelating agents
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine
In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen. ...
bridges (=CH−). The parent of porphyrin is porphine
Porphine or porphin is an organic chemical compound with formula . The molecule, which is flat, consists of four pyrrole-like rings joined by four methine (= C H−) groups to form a larger macrocycle ring, which makes it the simplest of the t ...
, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
. One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
word πορφύρα (''porphyra''), meaning ''purple''.
Complexes of porphyrins
Concomitant with the displacement of two N-''H'' protons, porphyrins bind metal ions in the N4 "pocket". The metalion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown:
:H2porphyrin + Lnsup>2+ → M(porphyrinate)Ln−4 + 4 L + 2 H+, where M = metal ion and L = a ligand
The insertion of the metal center is slow in the absence of catalysts. In nature, these catalysts (enzymes) are called chelatase
In biochemistry, chelatases are enzymes that catalyze the insertion ("metalation") of naturally occurring tetrapyrroles. Many tetrapyrrole-based cofactors exist in nature including hemes, chlorophylls, and vitamin B12. These metallo cofactors a ...
s. When there is no metal ion (or atom) bound to the nitrogens in the center, then the compounds are called ''free porphine'' or ''free porphyrin''. If they are bonded to a metal in the center, then they are ''bound''. A porphyrin with an iron atom of the type found in myoglobin, hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
, or certain cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of bi ...
s is called heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
.
Metal complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es derived from porphyrins, often called metalloporphyins, occur naturally. One of the best-known families of porphyrin complexes is heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
, the pigment in red blood cell
A blood cell, also called a hematopoietic cell, hemocyte, or hematocyte, is a cell produced through hematopoiesis and found mainly in the blood. Major types of blood cells include red blood cells (erythrocytes), white blood cells (leukocytes) ...
s, a cofactor of the protein hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
. Porphin
Porphine or porphin is an organic chemical compound with formula . The molecule, which is flat, consists of four pyrrole-like rings joined by four methine (= C H−) groups to form a larger macrocycle ring, which makes it the simplest of the tet ...
is the simplest porphyrin, a rare compound of theoretical interest.
protoporphyrin IX
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not solu ...
are common in nature, the precursor to heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
s.
File:H2octaethylporphyrin.png , Octaethylporphyrin
Octaethylporphyrin (H2OEP) is an organic compound that is a relative of naturally occurring heme pigments. The compound is used in the preparation of models for the prosthetic group in heme proteins. It is a dark purple solid that is soluble in ...
(H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
File:H2TPP.png, Tetraphenylporphyrin
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin ...
(H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methyne centers are occupied by phenyl groups.
File:Heme b.svg, Simplified view of heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
, a complex of a protoporphyrin IX.
Ancient porphyrins
A geoporphyrin, also known as a petroporphyrin, is a porphyrin of geologic origin. They can occur in crude oil,oil shale
Oil shale is an organic-rich fine-grained sedimentary rock containing kerogen (a solid mixture of organic chemical compounds) from which liquid hydrocarbons can be produced. In addition to kerogen, general composition of oil shales constitut ...
, coal, or sedimentary rocks. Abelsonite
Abelsonite is a nickel porphyrin mineral with chemical formula C31H32N4Ni. It was discovered in 1969 in the U.S. State of Utah and described in 1975. The mineral is named after geochemist Philip H. Abelson. It is the only known crystalline geo ...
is possibly the only geoporphyrin mineral, as it is rare for porphyrins to occur in isolation and form crystals.
The field of organic geochemistry
Organic geochemistry is the study of the impacts and processes that organisms have had on the Earth. It is mainly concerned with the composition and mode of origin of organic matter in rocks and in bodies of water. The study of organic geochemistr ...
had its origins in the isolation of porphyrins from petroleum. This finding helped establish the biological origins of petroleum. Petroleum is sometimes "fingerprinted" by analysis of trace amounts of nickel and vanadyl
The vanadyl or oxovanadium(IV) cation, VO2+, is a functional group that is common in the coordination chemistry of vanadium. Complexes containing this functional group are characteristically blue and paramagnetic. A triple bond is proposed to ex ...
porphyrins.
Biosynthesis
In non-photosynthetic eukaryotes such as animals, insects, fungi, and protozoa, as well as the α-proteobacteria group of bacteria, thecommitted step
In enzymology, the committed step (also known as the ''first'' committed step) is an effectively irreversible enzymatic reaction that occurs at a branch point during the biosynthesis of some molecules.
As the name implies, after this step, the m ...
for porphyrin biosynthesis is the formation of δ-aminolevulinic acid
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in ...
(δ-ALA, 5-ALA or dALA) by the reaction of the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
with succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A.
Sources
It is an important intermediate in the citric acid cycle, where it is synthesized from α-ketoglutarate by α-ketoglutarate d ...
from the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
. In plants
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclude ...
, algae, bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
(except for the α-proteobacteria group) and archaea, it is produced from glutamic acid via glutamyl-tRNA and glutamate-1-semialdehyde
Glutamate-1-semialdehyde is a molecule formed from by the reduction of tRNA bound glutamate, catalyzed by glutamyl-tRNA reductase. It is isomerized by glutamate-1-semialdehyde 2,1-aminomutase to give aminolevulinic acid in the biosynthesis of ...
. The enzymes involved in this pathway are glutamyl-tRNA synthetase, glutamyl-tRNA reductase
A glutamyl-tRNA reductase () is an enzyme that catalyzes the chemical reaction
:L-glutamate 1-semialdehyde + NADP+ + tRNAGlu \rightleftharpoons L-glutamyl-tRNAGlu + NADPH + H+
The 3 substrates of this enzyme are L-glutamate 1-semialdehyde, NA ...
, and glutamate-1-semialdehyde 2,1-aminomutase
In enzymology, a glutamate-1-semialdehyde 2,1-aminomutase () is an enzyme that catalyzes the chemical reaction
:L-glutamate 1-semialdehyde \rightleftharpoons 5-aminolevulinate
Hence, this enzyme has one substrate, L-glutamate-1-semialdehyde, ...
. This pathway is known as the C5 or Beale pathway.
Two molecules of dALA are then combined by porphobilinogen synthase
Aminolevulinic acid dehydratase (porphobilinogen synthase, or ALA dehydratase, or aminolevulinate dehydratase) is an enzyme () that in humans is encoded by the ''ALAD'' gene. Porphobilinogen synthase (or ALA dehydratase, or aminolevulinate de ...
to give porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.
The structure of the molecule can be described a ...
(PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases.
In the human body, deamination takes place primarily in the liver, however it can also occur in the kidney. In situations of ...
into hydroxymethyl bilane
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is ofte ...
(HMB), which is hydrolysed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
to form the circular tetrapyrrole uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens.
Structure
The molecular structure of uroporphy ...
. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not solu ...
is combined with iron to form heme. Bile pigments are the breakdown products of heme.
The following scheme summarizes the biosynthesis of porphyrins, with references by EC number and the OMIM
Online Mendelian Inheritance in Man (OMIM) is a continuously updated catalog of human genes and genetic disorders and traits, with a particular focus on the gene-phenotype relationship. , approximately 9,000 of the over 25,000 entries in OMIM ...
database. The porphyria
Porphyria is a group of liver disorders in which substances called porphyrins build up in the body, negatively affecting the skin or nervous system. The types that affect the nervous system are also known as acute porphyria, as symptoms are ...
associated with the deficiency of each enzyme is also shown:
Laboratory synthesis
A common synthesis for porphyrins is the Rothemund reaction, first reported in 1936, which is also the basis for more recent methods described by Adler and Longo. The general scheme is a condensation andoxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
process starting with pyrrole and an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
.
:
Applications
Photodynamic therapy
Porphyrins have been evaluated in the context ofphotodynamic therapy
Photodynamic therapy (PDT) is a form of phototherapy involving light and a photosensitizing chemical substance, used in conjunction with molecular oxygen to elicit cell death (phototoxicity).
PDT is popularly used in treating acne. It is used cl ...
(PDT) since they strongly absorb light, which is then converted to heat in the illuminated areas. This technique has been applied in macular degeneration
Macular degeneration, also known as age-related macular degeneration (AMD or ARMD), is a medical condition which may result in blurred or no vision in the center of the visual field. Early on there are often no symptoms. Over time, however, som ...
using verteporfin
Verteporfin (trade name Visudyne), a benzoporphyrin derivative, is a medication used as a photosensitizer for photodynamic therapy to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular dege ...
.
PDT is considered a noninvasive cancer treatment, involving the interaction between light of a determined frequency, a photo-sensitizer, and oxygen. This interaction produces the formation of a highly reactive oxygen species (ROS), usually singlet oxygen, as well as superoxide anion, free hydroxyl radical, or hydrogen peroxide. These high reactive oxygen species react with susceptible cellular organic biomolecules such as; lipids, aromatic amino acids, and nucleic acid heterocyclic bases, to produce oxidative radicals that damage the cell, possibly inducing apoptosis or even necrosis.
Toxicology
Heme biosynthesis is used asbiomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
in environmental toxicology studies. While excess production of porphyrins indicate organochlorine
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlo ...
exposure, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
inhibits ALA dehydratase enzyme.
Biological applications
Porphyrins have been investigated as possible anti-inflammatory agents and evaluated on their anti-cancer and anti-oxidant activity. Several porphyrin-peptide conjugates were found to have antiviral activity against HIV ''in vitro''.Potential applications
Biomimetic catalysis
Although not commercialized, metalloporphyrin complexes are widely studied as catalysts for the oxidation of organic compounds. Particularly popular for such laboratory research are complexes of ''meso''-tetraphenylporphyrin
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin ...
and octaethylporphyrin
Octaethylporphyrin (H2OEP) is an organic compound that is a relative of naturally occurring heme pigments. The compound is used in the preparation of models for the prosthetic group in heme proteins. It is a dark purple solid that is soluble in ...
. Complexes with Mn, Fe, and Co catalyze a variety of reactions of potential interest in organic synthesis. Some complexes emulate the action of various heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
enzymes such as cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various co ...
, lignin peroxidase. Metalloporphyrins are also studied as catalysts for water splitting, with the purpose of generating molecular hydrogen and oxygen for fuel cells.
Molecular electronics and sensors
Porphyrin-based compounds are of interest as possible components ofmolecular electronics
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. The unifying feature is use of mo ...
and photonics. Synthetic porphyrin dyes have been incorporated in prototype dye-sensitized solar cells
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a '' photoelectr ...
.
Metalloporphyrins have been investigated as sensors.
Phthalocyanine
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity.
It is composed of four isoindole units linked by a ring of nitrogen ato ...
s, which are structurally related to porphyrins, are used in commerce as dyes and catalysts, but porphyrins are not.
Supramolecular chemistry
 Porphyrins are often used to construct structures in
Porphyrins are often used to construct structures in supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
composed of four porphyrins. A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
Theoretical interest in aromaticity
Porphyrinoid macrocycles can show variable aromaticity. An Hückel aromatic porphyrin is porphycene.antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aroma ...
, Mobius aromatic, and non aromatic porphyrinoid macrocycles are known.
See also
* A porphyrin-related disease:porphyria
Porphyria is a group of liver disorders in which substances called porphyrins build up in the body, negatively affecting the skin or nervous system. The types that affect the nervous system are also known as acute porphyria, as symptoms are ...
* Porphyrin coordinated to iron: heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
* A heme-containing group of enzymes: Cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various co ...
* Porphyrin coordinated to magnesium: chlorophyll
* The one-carbon-shorter analogues: corrole
A corrole is an aromatic tetrapyrrole. The corrin ring is also present in cobalamin ( vitamin B12). The ring consists of nineteen carbon atoms, with four nitrogen atoms in the core of the molecule. In this sense, corrole is very similar to porph ...
s, including vitamin B12, which is coordinated to a cobalt
* Corphins, the highly reduced porphyrin coordinated to nickel that binds the Cofactor F430
F430 is the cofactor (sometimes called the coenzyme) of the enzyme methyl coenzyme M reductase (MCR).
MCR catalyzes the reaction that releases methane in the final step of methanogenesis:
: + HS–CoB → + CoB–S–S–CoM
It is found ...
active site in methyl coenzyme M reductase (MCR)
* Nitrogen-substituted porphyrins: phthalocyanine
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity.
It is composed of four isoindole units linked by a ring of nitrogen ato ...
Gallery
Related species
In nature
Several heterocycles related to porphyrins are found in nature, almost always bound to metal ions. These includeSynthetic
A benzoporphyrin is a porphyrin with a benzene ring fused to one of the pyrrole units. e.g.verteporfin
Verteporfin (trade name Visudyne), a benzoporphyrin derivative, is a medication used as a photosensitizer for photodynamic therapy to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular dege ...
is a benzoporphyrin derivative.
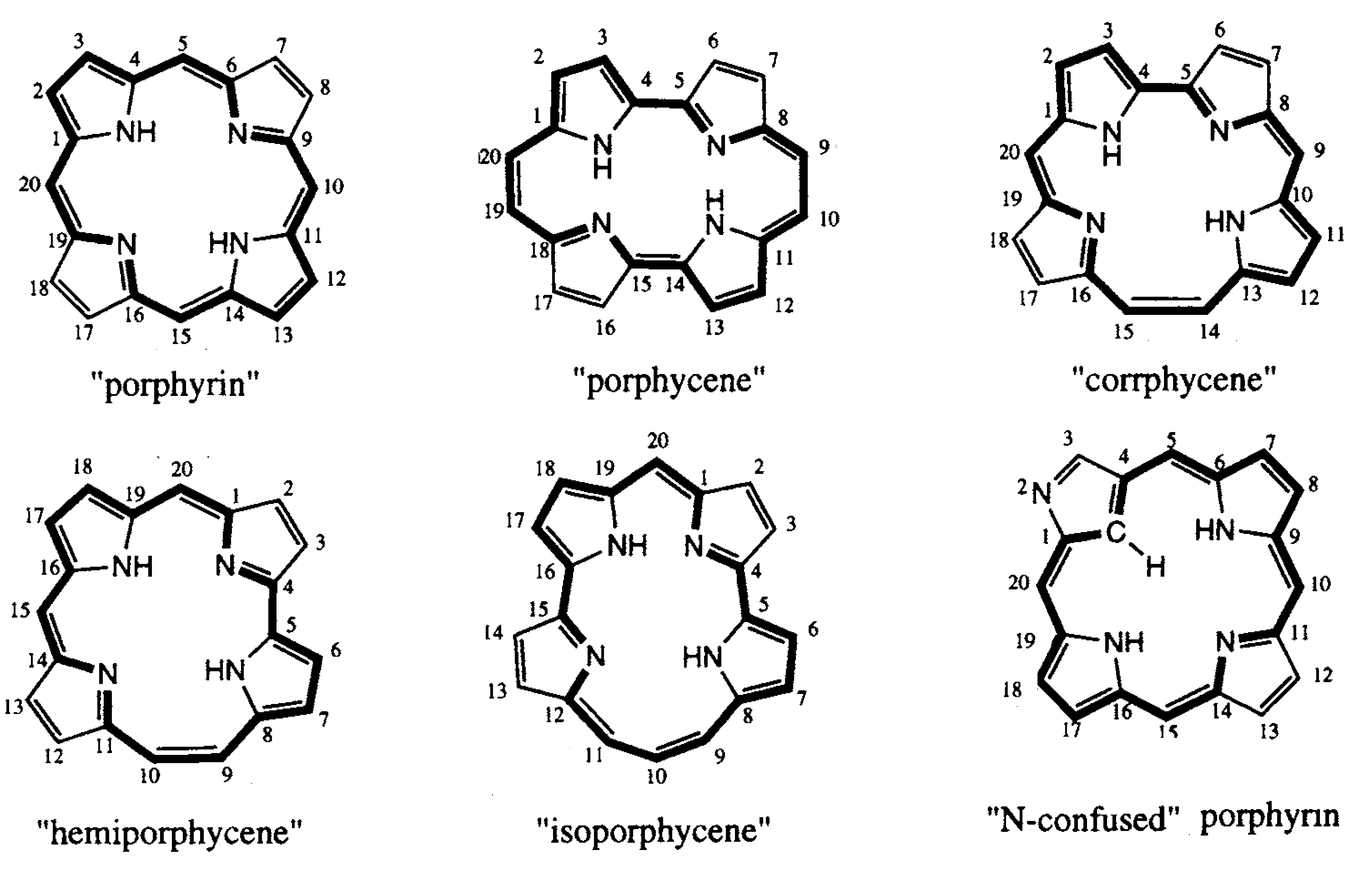
Non-natural porphyrin isomers
The first synthetic porphyrin isomer was reported by Emanual Vogel and coworkers in 1986. This isomer 8orphyrin-(2.0.2.0) is named as porphycene, and the central N4 Cavity forms a rectangle shape as shown in figure. Porphycenes showed interesting photophysical behavior and found versatile compound towards thephotodynamic therapy
Photodynamic therapy (PDT) is a form of phototherapy involving light and a photosensitizing chemical substance, used in conjunction with molecular oxygen to elicit cell death (phototoxicity).
PDT is popularly used in treating acne. It is used cl ...
. This inspired Vogel and Sessler to took up the challenge of preparing 8orphyrin-(2.1.0.1) and named it as Corrphycene or Porphycerin. The third porphyrin that is 8orphyrin-(2.1.1.0), was reported by Callot and Vogel-Sessler. Vogel and coworkers reported successful isolation of 8orphyrin-(3.0.1.0) or Isoporphycene. The Japanese scientist Furuta and Polish scientist Latos-Grażyński almost simultaneously reported the N-Confused porphyrins. The inversion of one of the pyrrolic subunits in the macrocyclic ring resulted to face one of the nitrogen atom outside of the core of the macrocycle.

References
External links
Journal of Porphyrins and Phthalocyanines
Porphynet – an informative site about porphyrins and related structures
{{Authority control Biomolecules Metabolism Photosynthetic pigments Chelating agents