Particle deposition on:
[Wikipedia]
[Google]
[Amazon]
 Particle deposition is the spontaneous attachment of particles to surfaces. The particles in question are normally
Particle deposition is the spontaneous attachment of particles to surfaces. The particles in question are normally
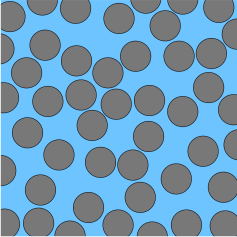 The blocking process has been studied in detail in terms of the '' random sequential adsorption'' (RSA) model.J. W. Evans, Rev. Mod. Phys. 65 (1993) 1281-1329. The simplest RSA model related to deposition of spherical particles considers irreversible adsorption of circular disks. One disk after another is placed randomly at a surface. Once a disk is placed, it sticks at the same spot, and cannot be removed. When an attempt to deposit a disk would result in an overlap with an already deposited disk, this attempt is rejected. Within this model, the surface is initially filled rapidly, but the more one approaches saturation the slower the surface is being filled. Within the RSA model, saturation is referred to as jamming. For circular disks, jamming occurs at a coverage of 0.547. When the depositing particles are polydisperse, much higher surface coverage can be reached, since the small particles will be able to deposit into the holes in between the larger deposited particles. On the other hand, rod like particles may lead to much smaller coverage, since a few misaligned rods may block a large portion of the surface.
Since the repulsion between particles in aqueous suspensions originates from electric double layer forces, the presence of salt has an important effect on surface blocking. For small particles and low salt, the diffuse layer will extend far beyond the particle, and thus create an exclusion zone around it. Therefore, the surface will be blocked at a much lower coverage than what would be expected based on the RSA model.M. R. Bohmer, E. A. van der Zeeuw, G. J. M. Koper, J. Colloid Interface Sci. 197 (1998) 242-250. At higher salt and for larger particles, this effect is less important, and the deposition can be well described by the RSA model.
The blocking process has been studied in detail in terms of the '' random sequential adsorption'' (RSA) model.J. W. Evans, Rev. Mod. Phys. 65 (1993) 1281-1329. The simplest RSA model related to deposition of spherical particles considers irreversible adsorption of circular disks. One disk after another is placed randomly at a surface. Once a disk is placed, it sticks at the same spot, and cannot be removed. When an attempt to deposit a disk would result in an overlap with an already deposited disk, this attempt is rejected. Within this model, the surface is initially filled rapidly, but the more one approaches saturation the slower the surface is being filled. Within the RSA model, saturation is referred to as jamming. For circular disks, jamming occurs at a coverage of 0.547. When the depositing particles are polydisperse, much higher surface coverage can be reached, since the small particles will be able to deposit into the holes in between the larger deposited particles. On the other hand, rod like particles may lead to much smaller coverage, since a few misaligned rods may block a large portion of the surface.
Since the repulsion between particles in aqueous suspensions originates from electric double layer forces, the presence of salt has an important effect on surface blocking. For small particles and low salt, the diffuse layer will extend far beyond the particle, and thus create an exclusion zone around it. Therefore, the surface will be blocked at a much lower coverage than what would be expected based on the RSA model.M. R. Bohmer, E. A. van der Zeeuw, G. J. M. Koper, J. Colloid Interface Sci. 197 (1998) 242-250. At higher salt and for larger particles, this effect is less important, and the deposition can be well described by the RSA model.
 Particle deposition is the spontaneous attachment of particles to surfaces. The particles in question are normally
Particle deposition is the spontaneous attachment of particles to surfaces. The particles in question are normally colloidal particles
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
, while the surfaces involved may be planar, curved, or may represent particles much larger in size than the depositing ones (e.g., sand grains). Deposition processes may be triggered by appropriate hydrodynamic flow conditions and favorable particle-surface interactions. Depositing particles may just form a monolayer which further inhibits additional particle deposition, and thereby one refers to ''surface blocking''. Initially attached particles may also serve as seeds for further particle deposition, which leads to the formation of thicker particle deposits, and this process is termed as ''surface ripening'' or ''fouling''. While deposition processes are normally irreversible, initially deposited particles may also detach. The latter process is known as ''particle release'' and is often triggered by the addition of appropriate chemicals or a modification in flow conditions.
Microorganisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
may deposit to surfaces in a similar fashion as colloidal particles. When macromolecules, such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s or polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are ...
s attach to surfaces, one rather calls this process adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
. While adsorption of macromolecules largely resembles particle deposition, macromolecules may substantially deform during adsorption. The present article mainly deals with particle deposition from liquids, but similar process occurs when aerosol
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of anthropogen ...
s or dust
Dust is made of fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian process), volcanic eruptions, and pollution. Dust in ...
deposit from the gas phase.
Initial stages
A particle may diffuse to a surface in quiescent conditions, but this process is inefficient as a thick depletion layer develops, which leads to a progressive slowing down of the deposition. When particle deposition is efficient, it proceeds almost exclusively in a system under flow. In such conditions, the hydrodynamic flow will transport the particles close to the surface. Once a particle is situated close to the surface, it will attach spontaneously, when the particle-surface interactions are attractive. In this situation, one refers to ''favorable deposition conditions''. When the interaction is repulsive at larger distances, but attractive at shorter distances, deposition will still occur but it will be slowed down. One refers to ''unfavorable deposition conditions'' here. The initial stages of the deposition process can be described with the rate equationW. B. Russel, D. A. Saville, W. R. Schowalter,''Colloidal Dispersions'',Cambridge University Press, 1989. : where ; is the number density of deposited particles, is the time, the particle number concentration, and the deposition rate coefficient. The rate coefficient depends on the flow velocity, flow geometry, and the interaction potential of the depositing particle with the substrate. In many situations, this potential can be approximated by a superposition of attractivevan der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and ...
s and repulsive electrical double layer forces and can be described by DLVO theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium ...
. When the charge of the particles is of the same sign as the substrate, deposition will be favorable at high salt levels, while it will be unfavorable at lower salt levels. When the charge of the particles is of the opposite sign as the substrate, deposition is favorable for all salt levels, and one observes a small enhancement of the deposition rate with decreasing salt level due to attractive electrostatic double layer forces. Initial stages of the deposition process are relatively similar to the early stages of particle heteroaggregation, whereby one of the particles is much larger than the other.
Blocking
When depositing particles repel each other, the deposition will stop by the time when enough particles have deposited. At one point, such a surface layer will repel any particles that may still make attempts to deposit. The surface is said to be ''saturated'' or ''blocked'' by the deposited particles. The blocking process can be described by the following equationM. Elimelech, J. Gregory, X. Jia, R. Williams, ''Particle Deposition and Aggregation: Measurement, Modelling and Simulation'', Butterworth-Heinemann, 1998. : where is the surface blocking function. When there are no deposited particles, and . With increasing number density of deposited particles, the blocking function decreases. The surface saturates at and . The simplest blocking function isZ. Adamczyk, Adv. Colloid Interface Sci. 2003, 100, 267-347. : and it is referred to as the Langmuir blocking function, as it is related to theLangmuir isotherm
The Langmuir adsorption model explains adsorption by assuming an adsorbate behaves as an ideal gas at isothermal conditions. According to the model, adsorption and desorption are reversible processes. This model even explains the effect of pressu ...
.
 The blocking process has been studied in detail in terms of the '' random sequential adsorption'' (RSA) model.J. W. Evans, Rev. Mod. Phys. 65 (1993) 1281-1329. The simplest RSA model related to deposition of spherical particles considers irreversible adsorption of circular disks. One disk after another is placed randomly at a surface. Once a disk is placed, it sticks at the same spot, and cannot be removed. When an attempt to deposit a disk would result in an overlap with an already deposited disk, this attempt is rejected. Within this model, the surface is initially filled rapidly, but the more one approaches saturation the slower the surface is being filled. Within the RSA model, saturation is referred to as jamming. For circular disks, jamming occurs at a coverage of 0.547. When the depositing particles are polydisperse, much higher surface coverage can be reached, since the small particles will be able to deposit into the holes in between the larger deposited particles. On the other hand, rod like particles may lead to much smaller coverage, since a few misaligned rods may block a large portion of the surface.
Since the repulsion between particles in aqueous suspensions originates from electric double layer forces, the presence of salt has an important effect on surface blocking. For small particles and low salt, the diffuse layer will extend far beyond the particle, and thus create an exclusion zone around it. Therefore, the surface will be blocked at a much lower coverage than what would be expected based on the RSA model.M. R. Bohmer, E. A. van der Zeeuw, G. J. M. Koper, J. Colloid Interface Sci. 197 (1998) 242-250. At higher salt and for larger particles, this effect is less important, and the deposition can be well described by the RSA model.
The blocking process has been studied in detail in terms of the '' random sequential adsorption'' (RSA) model.J. W. Evans, Rev. Mod. Phys. 65 (1993) 1281-1329. The simplest RSA model related to deposition of spherical particles considers irreversible adsorption of circular disks. One disk after another is placed randomly at a surface. Once a disk is placed, it sticks at the same spot, and cannot be removed. When an attempt to deposit a disk would result in an overlap with an already deposited disk, this attempt is rejected. Within this model, the surface is initially filled rapidly, but the more one approaches saturation the slower the surface is being filled. Within the RSA model, saturation is referred to as jamming. For circular disks, jamming occurs at a coverage of 0.547. When the depositing particles are polydisperse, much higher surface coverage can be reached, since the small particles will be able to deposit into the holes in between the larger deposited particles. On the other hand, rod like particles may lead to much smaller coverage, since a few misaligned rods may block a large portion of the surface.
Since the repulsion between particles in aqueous suspensions originates from electric double layer forces, the presence of salt has an important effect on surface blocking. For small particles and low salt, the diffuse layer will extend far beyond the particle, and thus create an exclusion zone around it. Therefore, the surface will be blocked at a much lower coverage than what would be expected based on the RSA model.M. R. Bohmer, E. A. van der Zeeuw, G. J. M. Koper, J. Colloid Interface Sci. 197 (1998) 242-250. At higher salt and for larger particles, this effect is less important, and the deposition can be well described by the RSA model.
Ripening
When the depositing particles attract each other, they will deposit and aggregate at the same time. This situation will result in a porous layer made of particle aggregates at the surface, and is referred to as ripening. The porosity of this layer will depend whether the particle aggregation process is fast or slow. Slow aggregation will lead to a more compact layer, while fast aggregation to a more porous one. The structure of the layer will resemble the structure of the aggregates formed in the later stages of the aggregation process.Experimental techniques
Particle deposition can be followed by various experimental techniques. Direct observation of deposited particles is possible with anoptical microscope
The optical microscope, also referred to as a light microscope, is a type of microscope that commonly uses visible light and a system of lenses to generate magnified images of small objects. Optical microscopes are the oldest design of micro ...
, scanning electron microscope, or the atomic force microscope
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the op ...
. Optical microscopy has the advantage that the deposition of particles can be followed in real time by video techniques and the sequence of images can be analyzed quantitatively. On the other hand, the resolution of optical microscopy requires that the particle size investigated exceeds at least 100 nm.
An alternative is to use surface sensitive techniques to follow particle deposition, such as reflectivity
The reflectance of the surface of a material is its effectiveness in reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic ...
, ellipsometry
Ellipsometry is an optical technique for investigating the dielectric properties (complex refractive index or dielectric function) of thin films. Ellipsometry measures the change of polarization upon reflection or transmission and compares it t ...
, surface plasmon resonance
Surface plasmon resonance (SPR) is the resonant oscillation of conduction electrons at the interface between negative and positive permittivity material in a particle stimulated by incident light. SPR is the basis of many standard tools for measu ...
, or quartz crystal microbalance A quartz crystal microbalance (QCM) (also known as ''quartz microbalance'' (QMB), sometimes also as ''quartz crystal nanobalance'' (QCN)) measures a mass variation per unit area by measuring the change in frequency of a quartz crystal resonator. The ...
. These techniques can provide information on the amount of particles deposited as a function of time with good accuracy, but they do not permit to obtain information concerning the lateral arrangement of the particles.
Another approach to study particle deposition is to investigate their transport in a chromatographic
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
column. The column is packed with large particles or with a porous medium to be investigated. Subsequently, the column is flushed with the solvent to be investigated, and the suspension of the small particles is injected at the column inlet. The particles are detected at the outlet with a standard chromatographic detector. When particles deposit in the porous medium, they will not arrive at the outlet, and from the observed difference the deposition rate coefficient can be inferred.
Relevance
Particle deposition occurs in numerous natural and industrial systems. Few examples are given below. *Coating
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. Pow ...
s and surface functionalization
Surface modification is the act of modifying the surface of a material by bringing physical, chemical or biological characteristics different from the ones originally found on the surface of a material.
This modification is usually made to solid m ...
. Paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s and adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advant ...
s often are concentrated suspensions of colloidal particles, and in order to adhere well to the surface the particles must deposit to the surface in question. Deposits of a monolayer of colloidal particles can be used to pattern the surface on a μm or nm scale, a process referred to as ''colloidal lithography''.
* Filters
Filter, filtering or filters may refer to:
Science and technology
Computing
* Filter (higher-order function), in functional programming
* Filter (software), a computer program to process a data stream
* Filter (video), a software component that ...
and filtration membranes. When particle deposit to filters or filtration membranes, they lead to pore clogging a membrane fouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling) or a non-living substance (inorganic or organic). Fouling is usually distinguished from other surf ...
.X. Zhu, M. Elimelech, Environ. Sci. Technol. 31 (1997) 3654-3662. When designing well functioning membranes, particle deposition must be avoided, and proper functionalization of the membranes is essential.
* Deposition of microorganisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
. Microorganisms may deposit similarly to colloidal particles. This deposition is a desired phenomenon in subsurface waters, as the aquifer filters out eventually injected microorganisms during the recharge of aquifers.S. F. Simoni, H. Harms, T. N. P. Bosma, A. J. B. Zehnder, Environ. Sci. Technol. 32 (1998) 2100-2105 On the other hand, such deposition is highly undesired at the surface of human teeth as it represent the origin of dental plaque
Dental plaque is a biofilm of microorganisms (mostly bacteria, but also fungi) that grows on surfaces within the mouth. It is a sticky colorless deposit at first, but when it forms tartar, it is often brown or pale yellow. It is commonly found be ...
s. Deposition of microorganisms is also relevant in the formation of biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular po ...
s.
See also
*Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
*Aerosol
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of anthropogen ...
*Biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular po ...
*Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
*DLVO theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium ...
* Double layer forces
*Electrical double layer
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The D ...
*Fouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling) or a non-living substance (inorganic or organic). Fouling is usually distinguished from other surf ...
*Nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
*Particle aggregation Particle agglomeration refers to formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, ...
* Polyelectrolyte adsorption
*Polymer adsorption Adsorption is the adhesion of ions or molecules onto the surface of another phase. Adsorption may occur via physisorption and chemisorption. Ions and molecules can adsorb to many types of surfaces including polymer surfaces. A polymer is a large mol ...
*Protein adsorption Adsorption (not to be mistaken for ''absorption'') is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of ...
* Random sequential adsorption
*Surface charge
Surface charge is a two-dimensional surface with non-zero electric charge. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge di ...
*van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and ...
References
{{DEFAULTSORT:Particle deposition Chemistry Materials science Colloidal chemistry