Oxocarbon anion on:
[Wikipedia]
[Google]
[Amazon]
 In
In
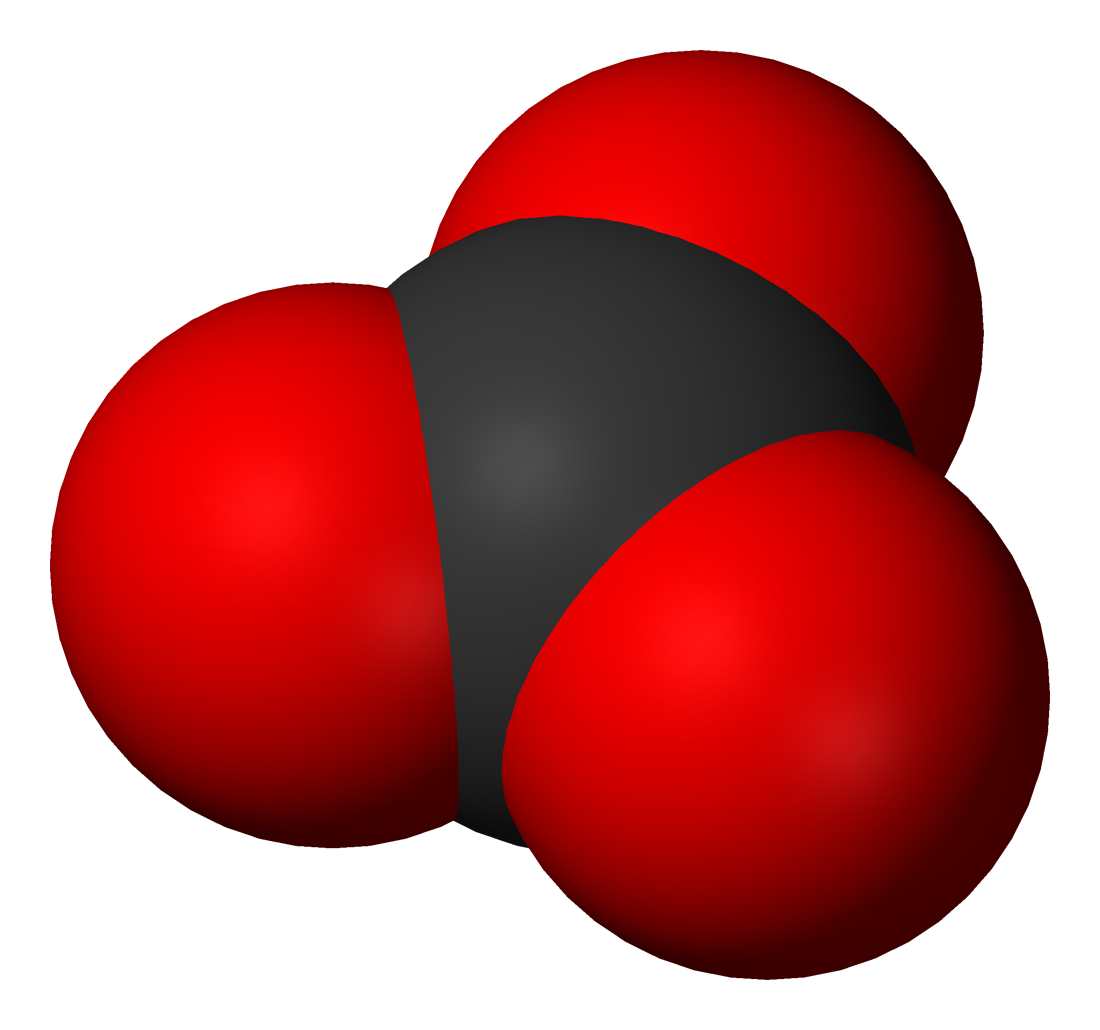 The carbonate ion has a trigonal planar structure,
The carbonate ion has a trigonal planar structure, 
 In each canonical form there are two single bonds one double bond. The three canonical forms contribute equally to the resonance hybrid, so the three bond C-O bonds have the same length.
In each canonical form there are two single bonds one double bond. The three canonical forms contribute equally to the resonance hybrid, so the three bond C-O bonds have the same length.
 With
With
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
, an oxocarbon anion is a negative ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
consisting solely of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
atoms, and therefore having the general formula for some integers ''x'', ''y'', and ''n''.
The most common oxocarbon anions are carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
, , and oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
, . There is however a large number of stable anions in this class, including several ones that have research or industrial use. There are also many unstable anions, like and , that have a fleeting existence during some chemical reactions; and many hypothetical species, like , that have been the subject of theoretical studies but have yet to be observed.
Stable oxocarbon anions form salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively c ...
with a large variety of cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s. Unstable anions may persist in very rarefied gaseous state, such as in interstellar cloud
An interstellar cloud is generally an accumulation of gas, plasma, and dust in our and other galaxies. Put differently, an interstellar cloud is a denser-than-average region of the interstellar medium, the matter and radiation that exists in the ...
s. Most oxocarbon anions have corresponding moieties in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, whose compounds are usually esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
. Thus, for example, the oxalate moiety occurs in the ester dimethyl oxalate .
Electronic structure of the carbonate ion
 The carbonate ion has a trigonal planar structure,
The carbonate ion has a trigonal planar structure, point group
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every ...
D3h. The three C-O bonds have the same length of 136 pm and the 3 O-C-O angles are 120°. The carbon atom has 4 pairs of valence electrons, which shows that the molecule obeys the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rul ...
. This is one factor that contributes to the high stability of the ion, which occurs in rocks such as limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms w ...
. The electronic structure is described by two main theories which are used to show how the 4 electron pairs are distributed in a molecule that only has 3 C-O bonds.
With valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
the electronic structure of the carbonate ion is a resonance hybrid of 3 canonical forms.
:
 In each canonical form there are two single bonds one double bond. The three canonical forms contribute equally to the resonance hybrid, so the three bond C-O bonds have the same length.
In each canonical form there are two single bonds one double bond. The three canonical forms contribute equally to the resonance hybrid, so the three bond C-O bonds have the same length.
molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molec ...
the 3-fold axis is designated as the z axis of the molecule. Three σ bonds are formed overlap of the s, px and py orbitals on the carbon atom with a p orbital on each oxygen atom. In addition, a delocalized π bond is made by overlap of the pz orbital on the carbon atom with the pz orbital on each oxygen atom which is perpendicular to the plane of the molecule.
Note that the same bonding schemes may be applied the nitrate ion
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insolubl ...
, NO3−, which is isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
with the carbonate ion.
Similarly, the two-fold symmetrical structure of a carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylat ...
group,, may be described as a resonance hybrid of two canonical forms in valence bond theory, or with 2 σ bonds and a delocalized π bond in molecular orbital theory.
Related compounds
Oxocarbon acids
An oxocarbon anion can be seen as the result of removing allproton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s from a corresponding acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
C''x''H''n''O''y''. Carbonate , for example, can be seen as the anion of carbonic acid H2CO3. Sometimes the "acid" is actually an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
or other species; this is the case, for example, of acetylenediolate that would yield acetylenediol C2H2O2. However, the anion is often more stable than the acid (as is the case for carbonate); and sometimes the acid is unknown or is expected to be extremely unstable (as is the case of methanetetracarboxylate
In chemistry, methanetetracarboxylate is a tetravalent anion with formula or C(COO−)4. It has four carboxylate groups attached to a central carbon atom; so it has the same carbon backbone as neopentane. It is an oxocarbon anion, that is, c ...
C(COO−)4).
Neutralized species
Every oxocarbon anion can be matched in principle to the electrically neutral (oroxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
) variant C''x''O''y'', an oxocarbon
In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide (). Many other stable (practically if not thermodynamica ...
(oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
of carbon) with the same composition and structure except for the negative charge. As a rule, however, these neutral oxocarbons are less stable than the corresponding anions. Thus, for example, the stable carbonate anion corresponds to the extremely unstable neutral carbon trioxide
Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups ''Cs'', ''D3h'', and ''C2v.'' The ''C2v'' state, consisting of a dioxirane, has been ...
CO3; oxalate correspond to the even less stable 1,2-dioxetanedione C2O4; and the stable croconate
Croconic acid or 4,5-dihydroxycyclopentenetrione is a chemical compound with formula or . It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitiv ...
anion corresponds to the neutral cyclopentanepentone C5O5, which has been detected only in trace amounts.
Reduced variants
Conversely, some oxocarbon anions can be reduced to yield other anions with the same structural formula but greater negative charge. Thus rhodizonate can be reduced to the tetrahydroxybenzoquinone (THBQ) anion and then to benzenehexolate . Haiyan Chen, Michel Armand, Matthieu Courty, Meng Jiang, Clare P. Grey, Franck Dolhem, Jean-Marie Tarascon, and Philippe Poizot (2009), "Lithium Salt of Tetrahydroxybenzoquinone: Toward the Development of a Sustainable Li-Ion Battery" ''J. Am. Chem. Soc.'', 131(25), pp. 8984–8988Acid anhydrides
An oxocarbon anion can also be associated with theanhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
of the corresponding acid. The latter would be another oxocarbon with formula C''x''O''y''−; namely, the acid minus water molecules H2O. The standard example is the connection between carbonate and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
CO2. The correspondence is not always well-defined since there may be several ways of performing this formal dehydration, including joining two or more anions to make an oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relati ...
or polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
. Unlike neutralization, this formal dehydration sometimes yields fairly stable oxocarbons, such as mellitic anhydride C12O9 from mellitate via mellitic acid
Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid. It crystallizes in fine silky ne ...
C12H6O12
Hydrogenated anions
For each oxocarbon anion there are in principle ''n''−1 partially hydrogenated anions with formulas , where ''k'' ranges from 1 to ''n''−1. These anions are generally indicated by the prefixes "hydrogen"-, "dihydrogen"-, "trihydrogen"-, etc. Some of them, however, have special names: hydrogencarbonate is commonly calledbicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemi ...
, and hydrogenoxalate is known as binoxalate.
The hydrogenated anions may be stable even if the fully protonated acid is not (as is the case of bicarbonate).
List of oxocarbon anions
Here is an incomplete list of the known or conjectured oxocarbon anions Several other oxocarbon anions have been detected in trace amounts, such as , a singly ionized version of rhodizonate. Richard B. Wyrwas and Caroline Chick Jarrold (2006), "Production of from Oligomerization of CO on Molybdenum Anions". ''J. Am. Chem. Soc.'' volume 128 issue 42, pages 13688–13689.See also
*Oxocarbon
In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide (). Many other stable (practically if not thermodynamica ...
* Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is a ...
* Sodium percarbonate (actually a carbonate perhydrate)
References
{{Reflist Oxyanions Oxocarbons