Organoiron chemistry on:
[Wikipedia]
[Google]
[Amazon]
Organoiron chemistry is the


 Compounds of the type η3-allyl)Fe(CO)4sup>+X− are allyl cation
Compounds of the type η3-allyl)Fe(CO)4sup>+X− are allyl cation

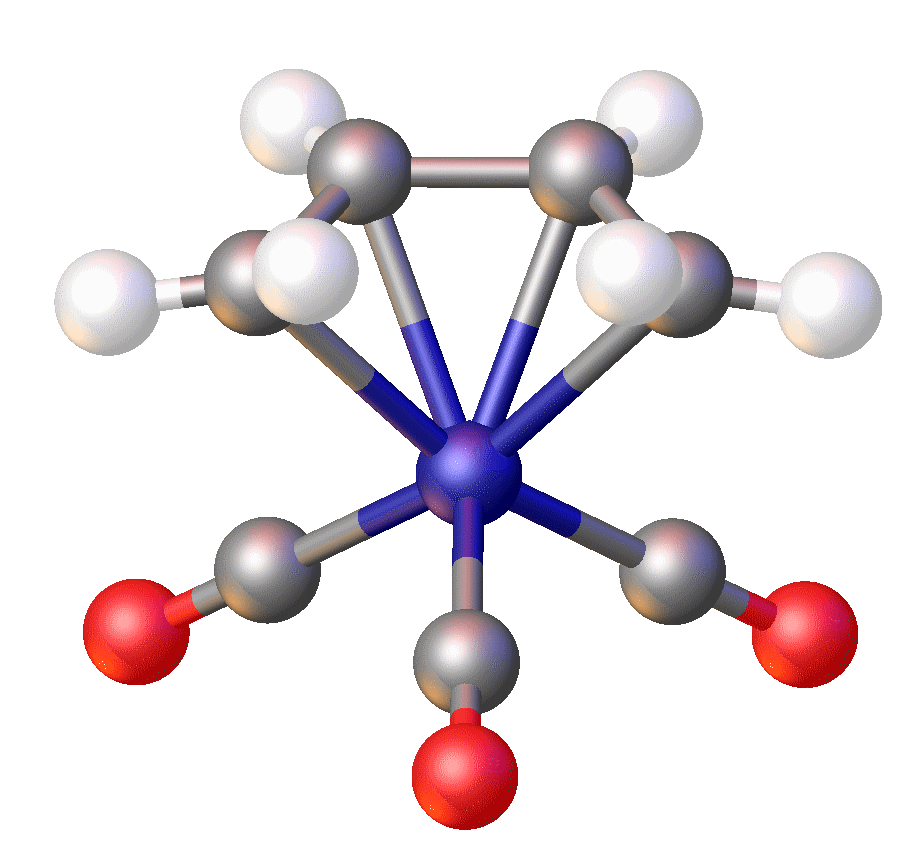
 In Fe(norbornyl)4, Fe(IV) is stabilized by an alkyl ligand that resists
In Fe(norbornyl)4, Fe(IV) is stabilized by an alkyl ligand that resists
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
of iron compounds containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
-to-iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
. Organoiron compounds are relevant in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
as reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s such as iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to ...
, diiron nonacarbonyl and disodium tetracarbonylferrate
Disodium tetracarbonylferrate is the organoiron compound with the formula Na2 e(CO)4 It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reag ...
. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and " greener" than other metals. Organoiron compounds feature a wide range of ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
s that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s, carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
, and cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
, but hard ligands such as amines are employed as well.
Iron(0) and more reduced states

Carbonyl complexes
Important iron carbonyls are the three neutral binary carbonyls,iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to ...
, diiron nonacarbonyl, and triiron dodecacarbonyl. One or more carbonyl ligands in these compounds can be replaced by a variety of other ligands including alkenes and phosphines. An iron(-II) complex, disodium tetracarbonylferrate
Disodium tetracarbonylferrate is the organoiron compound with the formula Na2 e(CO)4 It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reag ...
(Na2 e(CO)4, also known as "Collman's Reagent," is prepared by reducing iron pentacarbonyl with metallic sodium. The highly nucleophilic anionic reagent can be alkylated and carbonylated to give the acyl derivatives that undergo protonolysis to afford aldehydes:
:LiFe(CO)4(C(O)R) + H+ → RCHO (+ iron containing products)
Similar iron acyls can be accessed by treating iron pentacarbonyl with organolithium compounds:
:ArLi + Fe(CO)5 → LiFe(CO)4C(O)R
In this case, the carbanion attacks a CO ligand. In a complementary reaction, Collman's reagent can be used to convert acyl chlorides to aldehydes. Similar reactions can be achieved with Fe(CO)4sup>− salts.
Alkene-Fe(0)-CO derivatives

Monoalkenes
Iron pentacarbonyl reacts photochemically with alkenes to give Fe(CO)4(alkene).Diene-Fe(0)-CO derivatives
Iron diene complexes are usually prepared from Fe(CO)5 or Fe2(CO)9. Derivatives are known for common dienes like cyclohexadiene,norbornadiene
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distin ...
and cyclooctadiene
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH2)4(C2H2)2. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers a ...
, but even cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
can be stabilized. In the complex with butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two v ...
, the diene adopts a cis-conformation. Iron carbonyls are potential protective group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In ma ...
s for dienes, shielding them from hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
s and Diels-Alder reactions. Cyclobutadieneiron tricarbonyl
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive specie ...
is prepared from 3,4-dichlorocyclobutene and Fe2(CO)9.
Cyclohexadienes, many derived from Birch reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally ...
of aromatic compounds, form derivatives (diene)Fe(CO)3. The affinity of the Fe(CO)3 unit for conjugated dienes is manifested in the ability of iron carbonyls catalyse the isomerisation
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeri ...
s of 1,5-cyclooctadiene
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula , specifically .
There are three configurational isomers with this structure, that differ by the arrangement of the four C–C single bonds adjacent to the double bonds. Eac ...
to 1,3-cyclooctadiene. Cyclohexadiene complexes undergo hydride abstraction to give cyclohexadienyl cations, which add nucleophiles. Hydride abstraction from cyclohexadiene iron(0) complexes gives ferrous derivatives.
The enone complex (benzylideneacetone)iron tricarbonyl serves as a source of the Fe(CO)3 subunit and is employed to prepare other derivatives. It is used similarly to Fe2(CO)9.
Alkyne-Fe(0)-CO derivatives
Alkynes react with iron carbonyls to give a large variety of derivatives. Derivatives include ferroles (Fe2(C4R4)(CO)6), (p-quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds uch as benzene or naphthalene">benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= group ...
)Fe(CO)3, (cyclobutadiene)Fe(CO)3 and many others.
Tri- and polyene Fe(0) complexes
Stable iron-containing complexes with and without CO ligands are known for a wide variety of polyunsaturated hydrocarbons, e.g. cycloheptatriene,azulene
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that featur ...
, and bullvalene. In the case of cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
(COT), derivatives include Fe(COT)2, Fe3(COT)3, and several mixed COT-carbonyls (e.g. Fe(COT)(CO)3 and Fe2(COT)(CO)6).
144px, Bis(cyclooctatetraene)iron is an Fe(0) complex lacking CO ligands.
Iron(I) and iron(II)
As Fe(II) is a common oxidation state for Fe, many organoiron(II) compounds are known. Fe(I) compounds often feature Fe-Fe bonds, but exceptions occur, such as e(anthracene)2sup>−. :
Ferrocene and its derivatives
The rapid growth of organometallic chemistry in the 20th century can be traced to the discovery offerrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, ...
, a very stable compound which foreshadowed the synthesis of many related sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic deriv ...
s. Ferrocene is formed by reaction of sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are p ...
with iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water a ...
:
:2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
Ferrocene displays diverse reactivity localized on the cyclopentadienyl ligands, including Friedel–Crafts reactions and lithation. Some electrophilic functionalization reactions, however, proceed via initial attack at the Fe center to give the bent p2Fe–Zsup>+ species (which are formally Fe(IV)). For instance, HF:PF5 and Hg(OTFA)2, give isolable or spectroscopically observable complexes p2Fe–Hsup>+PF6– and Cp2Fe+–Hg–(OTFA)2, respectively.
Ferrocene is also a structurally unusual scaffold as illustrated by the popularity of ligands such as 1,1'-bis(diphenylphosphino)ferrocene, which are useful in catalysis. Treatment of ferrocene with aluminium trichloride and benzene gives the cation pFe(C6H6)sup>+. Oxidation of ferrocene gives the blue 17e species ferrocenium
Ferrocenium tetrafluoroborate is an organometallic compound with the formula e(C5H5)2F4. This salt is composed of the cation e(C5H5)2sup>+ and the tetrafluoroborate anion (). The related hexafluorophosphate is also a popular reagent with simi ...
. Derivatives of fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
can also act as a highly substituted cyclopentadienyl ligand.
Fp2, Fp−, and Fp+ and derivatives
Fe(CO)5 reacts withcyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca ...
to give the dinuclear Fe(I) species cyclopentadienyliron dicarbonyl dimer
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula ''η''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crysta ...
( eCp(CO)2sub>2), often abbreviated as Fp2. Pyrolysis of Fp2 gives the cuboidal cluster eCp(CO)sub>4.
Very hindered substituted cyclopentadienyl ligands can give an isolable monomeric Fe(I) species. For example, Cpi-PrFe(CO)2 (Cpi-Pr = i-Pr5C5) has been characterized crystallographically.
Reduction of Fp2 with sodium gives "NaFp", containing a potent nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
and precursor to many derivatives of the type CpFe(CO)2R. The derivative pCH2S(CH3)2sup>+ has been used in cyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolon ...
s. The complex Cp(CO2)Fe+(η2- vinyl ether]+ is a masked vinyl cation.
Fp-R compounds are prochiral, and studies have exploited the chiral derivatives CpFe(PPh3)(CO)acyl.
Alkyl, allyl, and aryl compounds
The simple peralkyl and peraryl complexes of iron are less numerous than are the Cp and CO derivatives. One example is tetramesityldiiron. Compounds of the type η3-allyl)Fe(CO)4sup>+X− are allyl cation
Compounds of the type η3-allyl)Fe(CO)4sup>+X− are allyl cation synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1 ...
s in allylic substitution An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.
In reaction conditions that favor a SN1 react ...
. In contrast, compounds of the type η5-C5H5)Fe(CO)2(CH2CH=CHR)possessing η1-allyl groups are analogous to main group allylmetal species (M = B, Si, Sn, etc.) and react with carbon electrophiles to give allylation products with SE2′ selectivity. Similarly, allenyl(cyclopentadienyliron) dicarbonyl complexes exhibit reactivity analogous to main group allenylmetal species and serve as nucleophilic propargyl synthons.
Sulfur and phosphorus derivatives
Complexes of the type Fe2(SR)2(CO)6 and Fe2(PR2)2(CO)6 form, usually by the reaction of thiols and secondary phosphines with iron carbonyls. The thiolates can also be obtained from the tetrahedrane Fe2S2(CO)6.Iron(III)
Some organoiron(III) compounds are prepared by oxidation of organoiron(II) compounds. A long-known example beingferrocenium
Ferrocenium tetrafluoroborate is an organometallic compound with the formula e(C5H5)2F4. This salt is composed of the cation e(C5H5)2sup>+ and the tetrafluoroborate anion (). The related hexafluorophosphate is also a popular reagent with simi ...
C5H5)2Fesup>+. Organoiron(III) porphyrin complexes are numerous.

Iron(IV)
beta-hydride elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo th ...
. Surprisingly, FeCy4, which is susceptible to beta-hydride elimination, has also been isolated and crystallographically characterized and is stable at –20 °C. The unexpected stability was attributed to stabilizing dispersive forces as well as conformational effects that disfavor beta-hydride elimination.
Two-electron oxidation of decamethylferrocene
Decamethylferrocene or bis(pentamethylcyclopentadienyl)iron(II) is a chemical compound with formula or . It is a sandwich compound, whose molecule has an iron(II) cation attached by coordination bonds between two pentamethylcyclopentadienyl a ...
gives the dication e(C5Me5)2sup>2+, which forms a carbonyl complex, e(C5Me5)2(CO)SbF6)2.
Organoiron compounds in organic synthesis and homogeneous catalysis
In industrial catalysis, iron complexes are seldom used in contrast tocobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
. Because of low cost and low toxicity of its salts, iron is attractive as a stoichiometric reagent. Some areas of investigation include:
* Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
and reduction, example catalyst Knölker complex.
* Cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M ...
s. Iron compounds such as Fe( acac)3 catalyze a wide range of cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M ...
s with one substrate an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
or alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
Grignard and the other substrate an aryl, alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
(vinyl), or acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC ...
organohalide
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlori ...
. In the related Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ...
the catalysts are based on palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
.
*Complexes derived from Schiff bases are active catalysts for olefin polymerization.Allan, L. E. N.; Shaver, M. P.; White, A. J. P. and Gibson, V. C., "Correlation of Metal Spin-State in alpha-Diimine Iron Catalysts with Polymerization Mechanism", Inorg. Chem., 2007, 46, 8963-8970.
Biochemistry
In the area of bioorganometallic chemistry, organoiron species are found at the active sites of the three hydrogenase enzymes as well as carbon monoxide dehydrogenase.Further reading
*References
{{ChemicalBondsToCarbon