Organogold chemistry on:
[Wikipedia]
[Google]
[Amazon]
Organogold chemistry is the study of compounds containing
 Gold cyanide compounds (MAu(CN)2) are of some importance to
Gold cyanide compounds (MAu(CN)2) are of some importance to

 This particular reaction demonstrated fantastic catalytic efficiency and would trigger a flurry of research into the use of phosphinegold(I) complexes for the activation C-C multiple bonds in the years to come. In spite of the lower stability of gold(III) complexes under catalytic conditions, simple AuCl3 was also found to be an efficient catalyst in some cases. For instance, Hashmi reported an AuCl3-catalyzed alkyne /
This particular reaction demonstrated fantastic catalytic efficiency and would trigger a flurry of research into the use of phosphinegold(I) complexes for the activation C-C multiple bonds in the years to come. In spite of the lower stability of gold(III) complexes under catalytic conditions, simple AuCl3 was also found to be an efficient catalyst in some cases. For instance, Hashmi reported an AuCl3-catalyzed alkyne /  Further mechanistic studies conclude that this is not a concerted transformation, but rather an initial alkyne hydroarylation followed by a series of non-obvious intramolecular rearrangements, concluding with a 6π electrocyclization and rearomatization.
Further mechanistic studies conclude that this is not a concerted transformation, but rather an initial alkyne hydroarylation followed by a series of non-obvious intramolecular rearrangements, concluding with a 6π electrocyclization and rearomatization.

 Although Echavarren first reported the preparation of chiral bisphosphinedigold(I) complexes for enantioselective gold catalysis proceeding via the typical pi-activation mechanism, an early, atypical example of enantioselective catalysis by gold was described by Hayashi and Ito in 1986. In this process,
Although Echavarren first reported the preparation of chiral bisphosphinedigold(I) complexes for enantioselective gold catalysis proceeding via the typical pi-activation mechanism, an early, atypical example of enantioselective catalysis by gold was described by Hayashi and Ito in 1986. In this process,  :
: In contrast to the other reactions described above, this reaction does not involve activation of a C-C double or triple bond by gold. In a simple mechanistic picture, gold(I) simultaneously coordinates to two phosphine ligands and the carbon isocyanate group which is then attacked by the carbonyl group. Further studies on the bonding mode of Au(I) indicate that this simple picture may have to be revised.
Heterogeneous gold catalysis is an older science. Gold is an attractive metal to use because of its stability against oxidation and its variety in morphology for instance gold cluster materials. Gold has been shown to be effective in low-temperature CO oxidation and acetylene hydrochlorination to vinyl chlorides. The exact nature of the catalytic site in this type of process is debated. The notion that gold can catalyse a reaction does not imply it is the only way. However, other metals can do the same job inexpensively, notably in recent years iron (see
In contrast to the other reactions described above, this reaction does not involve activation of a C-C double or triple bond by gold. In a simple mechanistic picture, gold(I) simultaneously coordinates to two phosphine ligands and the carbon isocyanate group which is then attacked by the carbonyl group. Further studies on the bonding mode of Au(I) indicate that this simple picture may have to be revised.
Heterogeneous gold catalysis is an older science. Gold is an attractive metal to use because of its stability against oxidation and its variety in morphology for instance gold cluster materials. Gold has been shown to be effective in low-temperature CO oxidation and acetylene hydrochlorination to vinyl chlorides. The exact nature of the catalytic site in this type of process is debated. The notion that gold can catalyse a reaction does not imply it is the only way. However, other metals can do the same job inexpensively, notably in recent years iron (see
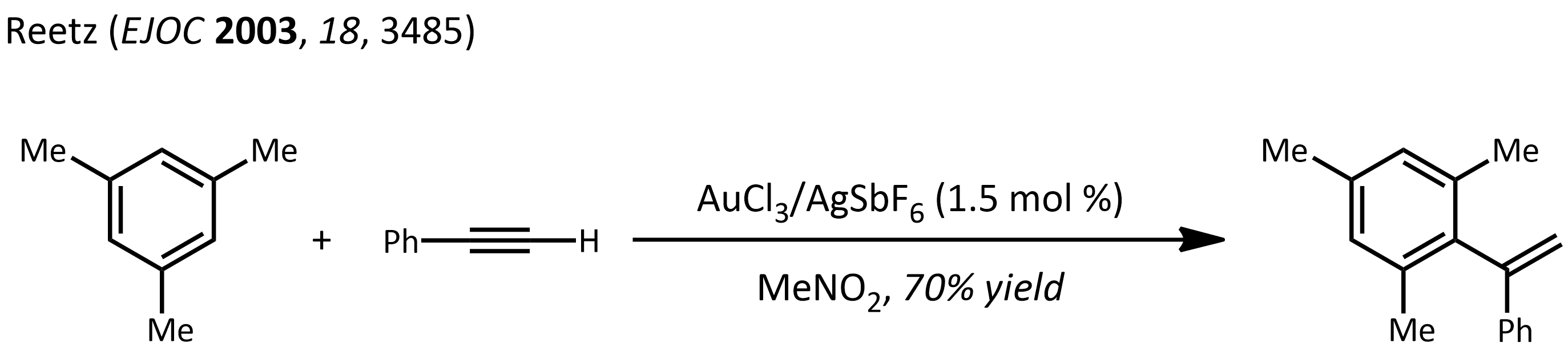 * Enyne cyclization, in particular cycloisomerization, one early example being a 5-exo-dig 1,6 enyne cycloisomerization:
:
* Enyne cyclization, in particular cycloisomerization, one early example being a 5-exo-dig 1,6 enyne cycloisomerization:
: *
*
gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
–carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s for organogold compounds are I with coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
2 and a linear molecular geometry
In chemistry, the linear molecular geometry describes the geometry around a central atom bonded to two other atoms (or ''ligands'') placed at a bond angle of 180°. Linear organic molecules, such as acetylene (), are often described by invoking ...
and III with CN = 4 and a square planar molecular geometry
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corne ...
.Elschenbroich, C. and Salzer, A. (1992) ''Organometallics : A Concise Introduction''. Wiley-VCH: Weinheim.
Gold(I)
Gold(I) complexes are 2-coordinate, linear,diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
, 14 electron species. They typically exist as adducts LAuR with as ligand L for instance a triphenylphosphine or an isocyanide. The ligand prevents reduction of Au(I) to metallic Au(0) with dimerization of the organic residue. Gold(I) can also exist as the aurate M uR2(the ate complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valence i ...
) whereby the cation is usually fitted with a complexing agent to improve stability. The AuR2− anion is also linear just as other M(d10) species such as Hg(Me)2 and Pd(Me)22+. Gold is known to form acetylide
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas and , where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure (where R is an organic side c ...
s (capable of forming polymeric structures), carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s and carbyne
In organic chemistry, a carbyne is a general term for any compound whose structure consists of an electrically neutral carbon atom connected by a single covalent bond and has three non-bonded electrons. The carbon atom has either one or thre ...
s. The classic method for the preparation of LAuR compounds is by reaction of a Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
with a gold(I) halide. A subsequent reaction with an organolithium
In organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, ...
R-Li forms the ate complex.
In a special group of compounds, an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
carbon atom acts as a bridge between two gold atoms. One such compound, (MesAu)5, is formed in a reaction between Au(CO)Cl and the mesityl
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzen ...
Grignard. Carbon can be coordinated with gold up to a value to 6. Compounds of the type C(AuL)4 are isolobal
In organometallic chemistry, the isolobal principle (more formally known as the isolobal analogy) is a strategy used to relate the structure of organic and inorganic molecular fragments in order to predict bonding properties of organometallic comp ...
with methane and those of type C(AuL)5+ isolobal with the methanium ion. These hypercoordinated organogold clusters are often stabilized by aurophilic interactions between the formally closed-shell gold centers.
: Gold cyanide compounds (MAu(CN)2) are of some importance to
Gold cyanide compounds (MAu(CN)2) are of some importance to gold cyanidation
Gold cyanidation (also known as the cyanide process or the MacArthur-Forrest process) is a hydrometallurgical technique for extracting gold from low-grade ore by converting the gold to a water-soluble coordination complex. It is the most commonly ...
, a process for the extraction of gold from low-grade ore. The carbon to metal bond in metal cyanides is usually ionic but evidence exists that the C-Au bonding in the gold cyanide ion is covalent.
Gold(III)
Gold(III) complexes are 4 coordinate, square planar,diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
, toxic, 16 electron species. When the formal coordination number is less than 4, ligands such as chlorine can make up for it by forming a bridging ligand. Intramolecular chelation is another strategy. In general gold(III) compounds are toxic and therefore less studied than gold(I). Monoarylgold(III) complexes are one well-studied class of complexes. They are often prepared by direct electrophilic auration of arenes by AuCl3. Homoleptic tetraalkylaurate(III) complexes (e.g. Li uMe4 are also well-characterized.
Gold catalysis
General considerations
Gold-catalyzed reactions fall into two major categories:heterogeneous catalysis
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Ph ...
including catalysts by gold nanoparticles
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either wine-red coloured (for spherical particles less than 100 nm) or blue/purple (for larger spherical particl ...
(e.g., Au/TiO2) and thiol-monolayer gold surfaces, and catalysts on alumina support, including alumina supported Au/CeO2. These catalysts have been investigated for industrially important processes like the oxidation of alcohols, oxidation of carbon monoxide (CO), and various selective hydrogenation reactions (e.g. butadiene to butene). Though often efficient and exhibiting useful or unique selectivities, there is considerable uncertainty with respect to the mechanism of processes catalyzed by various heterogeneous gold catalysts, even compared to other heterogeneous transition metal catalysts.
In contrast, homogeneous catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
with gold uses simple or ligand-bound gold(I) or gold(III) compounds that are soluble in organic solvents and is used for the synthesis of fine chemicals in organic chemistry. Binary gold halides and simple complexes, including gold(I) chloride
Gold(I) chloride is a compound of gold and chlorine with the chemical formula AuCl.
Preparation
Gold(I) chloride is prepared by thermal decomposition of gold(III) chloride.
Reactions
Although there is a region of stability at higher temperatures ...
, gold(III) chloride
Gold(III) chloride, traditionally called auric chloride, is a compound of gold and chlorine with the molecular formula . The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. Gold(III) c ...
, and chloroauric acid
Chloroauric acid is an inorganic compound with the chemical formula . It forms hydrates . Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar anion. Often chloroauric acid is handled as a soluti ...
, have been employed as complexes. These gold sources, however, quickly give rise to ill-defined and easily deactivated (via reduction to Au0) active catalysts in solution. The development of well-defined phosphine- or NHC-ligated gold(I) complexes was an important advance and led to significant increase in interest in the synthetic applications of gold catalysis. Ligated gold(I) complexes are typically prepared and stored as the bench-stable (but unreactive) chlorides, LAuCl, e.g., chloro(triphenylphosphine)gold(I)
Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula ( Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.
Preparation and structure
The complex is prep ...
, which are typically activated via halide abstraction with silver salts like AgOTf, AgBF4, or AgSbF6 to generate a cationic gold(I) species. Although the coordinatively unsaturated complex "LAu+" is notionally generated from a LAuCl/AgX mixture, the exact nature of the cationic gold species and the role of the silver salt remains somewhat contentious. The ''para''-nitrobenzoate, bistriflimide, and certain nitrile complexes represent catalytically active yet isolable silver-free precatalysts.
Cationic gold(I) forms π-complexes with alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
or alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
bonds, following the Dewar–Chatt–Duncanson model
The Dewar–Chatt–Duncanson model is a model in organometallic chemistry that explains the chemical bonding in transition metal alkene complexes. The model is named after Michael J. S. Dewar, Joseph Chatt and L. A. Duncanson.
The alkene donat ...
. Gold is certainly not the only metal showing this type of bonding and reactivity, several metal ions isolobal
In organometallic chemistry, the isolobal principle (more formally known as the isolobal analogy) is a strategy used to relate the structure of organic and inorganic molecular fragments in order to predict bonding properties of organometallic comp ...
with the simple proton (i.e., an empty s-orbital) do as well: for example, mercury(II) and platinum(II). Electrophilic ions and complexes such as these with a strong propensity to form π-complexes are generally known as pi(π)-acids (see also: cation–pi interaction).
Gold(I)-alkene and -alkyne complexes are electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
and susceptible toward nucleophilic attack. In oxymercuration
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate (AcO–Hg–OAc) in aqueous solution to yield the addition of ...
the resultant organomercurial species is generated stoichiometrically, and requires an additional step to liberate the product. In the case of gold, protonolysis
Protonolysis is the cleavage of a chemical bond by acids. Many examples are found in organometallic chemistry since the reaction requires polar Mδ+-Rδ- bonds, where δ+ and δ- signify partial positive and negative charges associated with the bon ...
of the Au-C bond closes the catalytic cycle, allowing the coordination of another substrate. Some practical advantages of gold(I) catalysis include: 1) air stability (due to the high oxidation potential of Au(I)), 2) tolerance towards adventitious moisture (due its low oxophilicity), and 3) relatively low toxicity compared to other pi-acids (e.g., Pt(II) and Hg(II)). Chemically, Au(I) complexes typically do not undergo oxidation to higher oxidation states, and Au(I)-alkyls and -vinyls are not susceptible to β hydride elimination.
:
Historical development
In 1976, Thomas and coworkers reported conversion ofphenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
I ...
to acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene p ...
using tetrachloroauric acid in a 37% yield. In this reaction gold(III) was used as a homogeneous catalyst replacing mercury in oxymercuration. This same study lists a published yield >150%, indicating catalysis that perhaps was not acknowledged by the chemists.
In 1991, Utimoto reacted gold(III) (NaAuCl4) with alkynes and water. Teles identified a major drawback of this method as Au(III) was rapidly reduced to catalytically dead metallic gold and in 1998 returned to the theme of ligand supported Au(I) for the same transformation:
: This particular reaction demonstrated fantastic catalytic efficiency and would trigger a flurry of research into the use of phosphinegold(I) complexes for the activation C-C multiple bonds in the years to come. In spite of the lower stability of gold(III) complexes under catalytic conditions, simple AuCl3 was also found to be an efficient catalyst in some cases. For instance, Hashmi reported an AuCl3-catalyzed alkyne /
This particular reaction demonstrated fantastic catalytic efficiency and would trigger a flurry of research into the use of phosphinegold(I) complexes for the activation C-C multiple bonds in the years to come. In spite of the lower stability of gold(III) complexes under catalytic conditions, simple AuCl3 was also found to be an efficient catalyst in some cases. For instance, Hashmi reported an AuCl3-catalyzed alkyne / furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
- a type of cycloaddition that does not ordinarily occur - for the synthesis of 2,3-disubstituted phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
s:
: Further mechanistic studies conclude that this is not a concerted transformation, but rather an initial alkyne hydroarylation followed by a series of non-obvious intramolecular rearrangements, concluding with a 6π electrocyclization and rearomatization.
Further mechanistic studies conclude that this is not a concerted transformation, but rather an initial alkyne hydroarylation followed by a series of non-obvious intramolecular rearrangements, concluding with a 6π electrocyclization and rearomatization.
Relativistic effects
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of ...
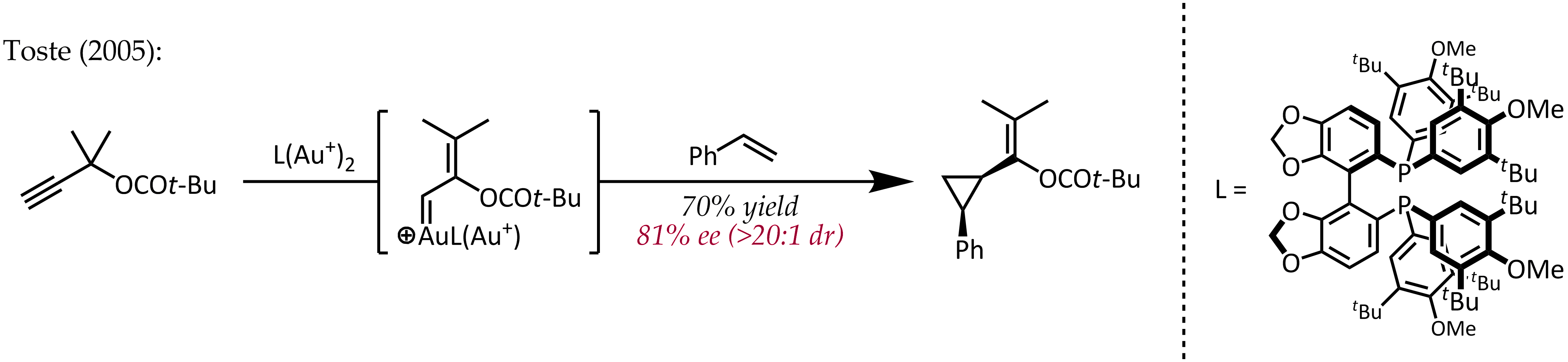
are significant in organogold chemistry due to the large nuclear charge of the metal (''Z'' = 79). As a consequence of relativistically expanded 5''d'' orbitals, the LAu fragment can stabilize a neighboring carbocation via electron donation into the empty ''p''-type orbital. Thus, in addition to their expected carbocation-like reactivity, these cations also exhibit significant carbene character, a property that has been exploited in catalytic transformations such as cyclopropanation and C-H insertion. Propargyl esters can serve as precursors for cationic gold-vinylcarbene intermediates, which can react with alkenes in a concerted manner to afford the cyclopropanation product. The use of a chiral ligand ( (''R'')-DTBM-SEGPHOS) resulted in good to excellent levels of enantioselectivity.

 Although Echavarren first reported the preparation of chiral bisphosphinedigold(I) complexes for enantioselective gold catalysis proceeding via the typical pi-activation mechanism, an early, atypical example of enantioselective catalysis by gold was described by Hayashi and Ito in 1986. In this process,
Although Echavarren first reported the preparation of chiral bisphosphinedigold(I) complexes for enantioselective gold catalysis proceeding via the typical pi-activation mechanism, an early, atypical example of enantioselective catalysis by gold was described by Hayashi and Ito in 1986. In this process, benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
and methyl isocyanoacetate undergo cyclization in the presence of a chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
ferrocenylphosphine ligand and a bis(isocyanide)gold(I) complex to form a chiral oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
. Since oxazolines can be hydrolyzed to provide a 1,2-aminoalcohol, this reaction constitutes the first example of a catalytic, asymmetric aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carb ...
.
 :
: In contrast to the other reactions described above, this reaction does not involve activation of a C-C double or triple bond by gold. In a simple mechanistic picture, gold(I) simultaneously coordinates to two phosphine ligands and the carbon isocyanate group which is then attacked by the carbonyl group. Further studies on the bonding mode of Au(I) indicate that this simple picture may have to be revised.
Heterogeneous gold catalysis is an older science. Gold is an attractive metal to use because of its stability against oxidation and its variety in morphology for instance gold cluster materials. Gold has been shown to be effective in low-temperature CO oxidation and acetylene hydrochlorination to vinyl chlorides. The exact nature of the catalytic site in this type of process is debated. The notion that gold can catalyse a reaction does not imply it is the only way. However, other metals can do the same job inexpensively, notably in recent years iron (see
In contrast to the other reactions described above, this reaction does not involve activation of a C-C double or triple bond by gold. In a simple mechanistic picture, gold(I) simultaneously coordinates to two phosphine ligands and the carbon isocyanate group which is then attacked by the carbonyl group. Further studies on the bonding mode of Au(I) indicate that this simple picture may have to be revised.
Heterogeneous gold catalysis is an older science. Gold is an attractive metal to use because of its stability against oxidation and its variety in morphology for instance gold cluster materials. Gold has been shown to be effective in low-temperature CO oxidation and acetylene hydrochlorination to vinyl chlorides. The exact nature of the catalytic site in this type of process is debated. The notion that gold can catalyse a reaction does not imply it is the only way. However, other metals can do the same job inexpensively, notably in recent years iron (see organoiron chemistry
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate ...
).
Gold catalyzed reactions
Gold catalyzes many organic transformations, usually carbon-carbon bond formation from Au(I), and C-X (X = O, N) bond formation from the Au(III) state, due to that ion's harder Lewis acidity. During the past decade, several studies have demonstrated that gold can efficiently catalyze C-C and C-heteroatom cross-coupling reactions that proceed through an Au(I)/Au(III) cycle. Hong C. Shen summarized homogeneous reactions forming cyclic compounds into 4 main categories: * heteroatomnucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
to unsaturated C-C bonds, especially to form small heterocycles (furans, pyrroles, thiophenes)
* Hydroarylation: basically a Friedel-Crafts reaction using metal-alkyne complexes. Example, the reaction of mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
with phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
I ...
:
: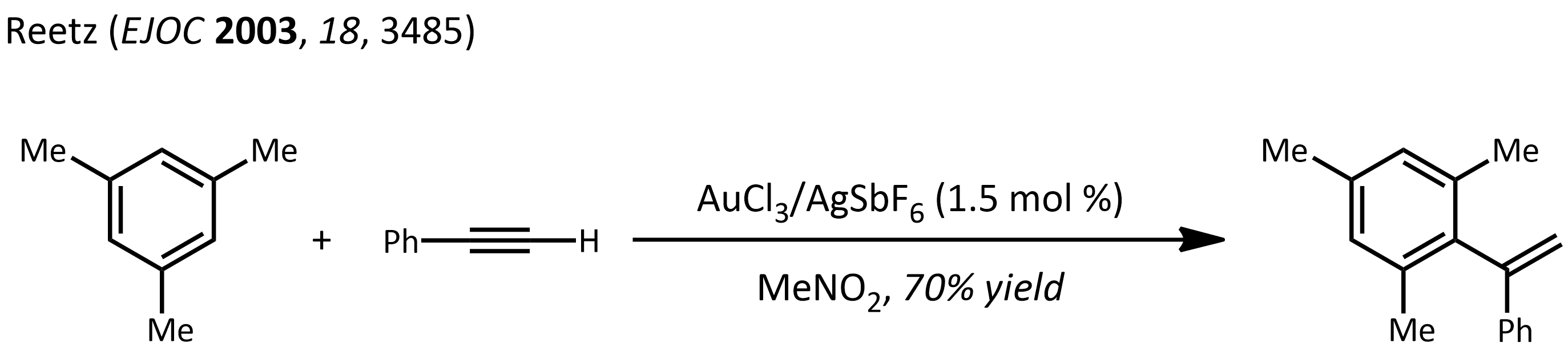 * Enyne cyclization, in particular cycloisomerization, one early example being a 5-exo-dig 1,6 enyne cycloisomerization:
:
* Enyne cyclization, in particular cycloisomerization, one early example being a 5-exo-dig 1,6 enyne cycloisomerization:
: *
* cycloaddition reaction
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
s with early example the cycloaddition of a nitrile oxide
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
with an alkyne.
Other reactions are the use of gold in C–H bond activation and aldol reactions. Gold also catalyses coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
s.
Limitations
While gold-catalyzed hydrofunctionalization of alkynes, allenes, and allylic alcohols occurs readily under comparatively mild conditions, unactivated alkene remain poor substrates in most cases, in large part due to the resistance of the intermediate alkylgold(I) complexes to protodeauration. The development of intermolecular gold-catalyzed transformations has also lagged behind the development of intramolecular ones.References
{{ChemicalBondsToCarbon *