Non-random two-liquid model on:
[Wikipedia]
[Google]
[Amazon]
 The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model that correlates the
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model that correlates the
It also shows, since three parameters are available, that multiple sets of solutions are possible.
 The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model that correlates the
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model that correlates the activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ...
s of a compound with its mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ex ...
s in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson that the local concentration around a molecule is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind and that with the molecules of the other kind . The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC
In statistical thermodynamics, UNIQUAC (a portmanteau of universal quasichemical) is an activity coefficient model used in description of phase equilibria.
The model is a so-called lattice model and has been derived from a first order approxi ...
model, and the group contribution model UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", '' ...
. These local-composition models are not thermodynamically consistent for a one-fluid model for a real mixture due to the assumption that the local composition around molecule ''i'' is independent of the local composition around molecule ''j''. This assumption is not true, as was shown by Flemr in 1976. However, they are consistent if a hypothetical two-liquid model is used.
Derivation
Like Wilson (1964), Renon & Prausnitz (1968) began with local composition theory, but instead of using the Flory–Huggins volumetric expression as Wilson did, they assumed local compositions followed : with a new "non-randomness" parameter α. The excessGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature an ...
was then determined to be
:.
Unlike Wilson's equation, this can predict partially miscible mixtures. However, the cross term, like Wohl's expansion, is more suitable for than , and experimental data is not always sufficiently plentiful to yield three meaningful values, so later attempts to extend Wilson's equation to partial miscibility (or to extend Guggenheim's quasichemical theory for nonrandom mixtures to Wilson's different-sized molecules) eventually yielded variants like UNIQUAC
In statistical thermodynamics, UNIQUAC (a portmanteau of universal quasichemical) is an activity coefficient model used in description of phase equilibria.
The model is a so-called lattice model and has been derived from a first order approxi ...
.
Equations for a binary mixture
For a binary mixture the following function are used: : with : Here, and are the dimensionless interaction parameters, which are related to the interaction energy parameters and by: : Here ''R'' is thegas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and ''T'' the absolute temperature, and ''Uij'' is the energy between molecular surface ''i'' and ''j''. ''Uii'' is the energy of evaporation. Here ''Uij'' has to be equal to ''Uji'', but is not necessary equal to .
The parameters and are the so-called non-randomness parameter, for which usually is set equal to . For a liquid, in which the local distribution is random around the center molecule, the parameter . In that case the equations reduce to the one-parameter Margules activity model The Margules activity model is a simple thermodynamic model for the excess Gibbs free energy of a liquid mixture introduced in 1895 by Max Margules. After Lewis had introduced the concept of the activity coefficient, the model could be used to der ...
:
:
In practice, is set to 0.2, 0.3 or 0.48. The latter value is frequently used for aqueous systems. The high value reflects the ordered structure caused by hydrogen bonds. However, in the description of liquid-liquid equilibria the non-randomness parameter is set to 0.2 to avoid wrong liquid-liquid description. In some cases a better phase equilibria description is obtained by setting . However this mathematical solution is impossible from a physical point of view, since no system can be more random than random ( =0). In general NRTL offers more flexibility in the description of phase equilibria than other activity models due to the extra non-randomness parameters. However, in practice this flexibility is reduced in order to avoid wrong equilibrium description outside the range of regressed data.
The limiting activity coefficients, also known as the activity coefficients at infinite dilution, are calculated by:
:
The expressions show that at the limiting activity coefficients are equal. This situation that occurs for molecules of equal size, but of different polarities.It also shows, since three parameters are available, that multiple sets of solutions are possible.
General equations
The general equation for for species in a mixture of components is: : with : : : There are several different equation forms for and , the most general of which are shown above.Temperature dependent parameters
To describe phase equilibria over a large temperature regime, i.e. larger than 50 K, the interaction parameter has to be made temperature dependent. Two formats are frequently used. The extendedAntoine equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presented ...
format:
:
Here the logarithmic and linear terms are mainly used in the description of liquid-liquid equilibria (miscibility gap A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible.
The IUPAC Gold Book defines ...
).
The other format is a second-order polynomial format:
:
Parameter determination
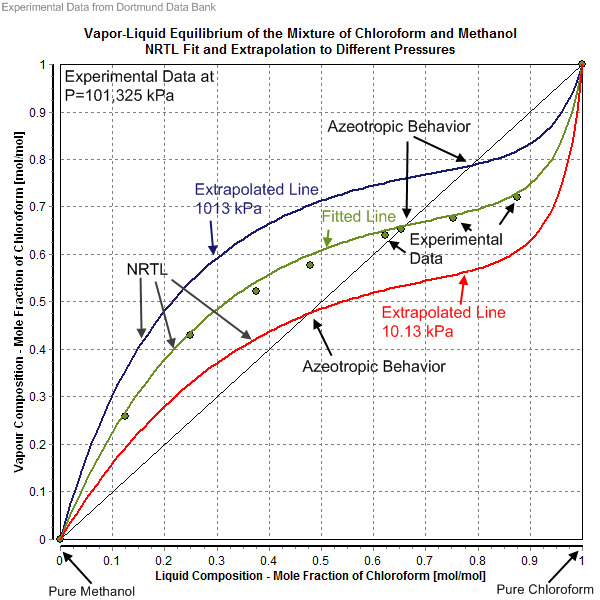
The NRTL parameters are fitted to activity coefficients that have been derived from experimentally determined phase equilibrium data (vapor–liquid, liquid–liquid, solid–liquid) as well as from heats of mixing. The source of the experimental data are often factual data banks like theDortmund Data Bank
The Dortmund Data Bank (short DDB) is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimizat ...
. Other options are direct experimental work and predicted activity coefficients with UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", '' ...
and similar models.
Noteworthy is that for the same liquid mixture several NRTL parameter sets might exist. The NRTL parameter set to use depends on the kind of phase equilibrium (i.e. solid–liquid (SL), liquid–liquid (LL), vapor–liquid (VL)). In the case of the description of a vapor–liquid equilibria it is necessary to know which saturated vapor pressure of the pure components was used and whether the gas phase was treated as an ideal or a real gas. Accurate saturated vapor pressure values are important in the determination or the description of an azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
. The gas fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas whic ...
coefficients are mostly set to unity (ideal gas assumption), but for vapor-liquid equilibria at high pressures (i.e. > 10 bar) an equation of state
In physics, chemistry, and thermodynamics, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or intern ...
is needed to calculate the gas fugacity coefficient for a real gas description.
Determination of NRTL parameters from LLE data is more complicated than parameter regression from VLE data as it involves solving isoactivity equations which are highly non-linear. In addition, parameters obtained from LLE may not always represent the real activity of components due to lack of knowledge on the activity values of components in the data regression. For this reason it is necessary to confirm the consistency of the obtained parameters in the whole range of compositions (including binary subsystems, experimental and calculated lie-lines, Hessian matrix, etc.).
Literature
{{reflist Physical chemistry Thermodynamic models Engineering thermodynamics