Mononitrogen monosulfide on:
[Wikipedia]
[Google]
[Amazon]
Sulfur mononitride is an
 Removal of byproducts leaves only N4S4 in
Removal of byproducts leaves only N4S4 in 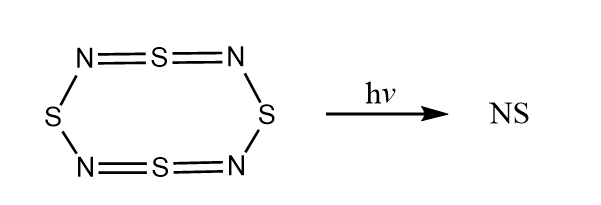

 ''Trans''-NSSN results from direct dimerization of NS.
''Trans''-NSSN results from direct dimerization of NS.
 N3S3 has been observed through photoelectron spectroscopy of vapors of the (SN)x, polymer, but has not yet been characterized further. Attempts to produce N3S3 by oxidation of PNS3N3] were unsuccessful. Its theorized that rapid dimerization to (N3S3)2 will disproportionate irreversibly to N4S4 and N2S2.
N3S3 has been observed through photoelectron spectroscopy of vapors of the (SN)x, polymer, but has not yet been characterized further. Attempts to produce N3S3 by oxidation of PNS3N3] were unsuccessful. Its theorized that rapid dimerization to (N3S3)2 will disproportionate irreversibly to N4S4 and N2S2.

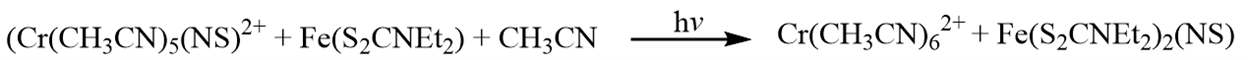 This was particularly significant as it was the first controlled and well-characterized reactivity of NS in solution. Further, it showed the potential for similar reactivity in known reactions with NO, such as use of this iron dithiocarbamate complex.
This was particularly significant as it was the first controlled and well-characterized reactivity of NS in solution. Further, it showed the potential for similar reactivity in known reactions with NO, such as use of this iron dithiocarbamate complex.
 The valence electrons of this compound match those of
The valence electrons of this compound match those of 

inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemis ...
with the molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
NS. It is the sulfur analogue of and isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
to the radical nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
, NO. It was initially detected in 1975, in outer space in giant molecular clouds and later the coma of comets. This spurred further laboratory studies of the compound. Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.
Synthesis
The NSradical
Radical may refer to:
Politics and ideology Politics
* Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe an ...
is a highly transient species, with a lifetime on the order of milliseconds, but it can be observed spectroscopically over short periods of time through several methods of generation. NS is too reactive to isolate as a solid or liquid, and has only been prepared as a vapor in low pressure or low-temperature matrices due to its tendency to rapidly oligomerize to more stable, diamagnetic species.
Discharge of nitrogen and sulfur vapor
Transmission of electric discharge through a glass tube with quartz windows containing a mixture of nitrogen and sulfur vapor (rigorously free of oxygen) results in the spectrum of emitted light gaining bands consistent with the formation of NS. Passing a mixture of gaseous N2 and S2Cl2 through the side arm of an absorption cell undergoing microwave discharge produces NS. Infrared diode laser spectroscopy taken using this method allowed for derivation of the equilibrium rotational constant, and therefore calculation of the equilibrium bond length as 1.4940 Å. With low pressure microwave discharge of elemental nitrogen and sulfur, followed by low temperature trapping in argon matrices, one obtains a mixture of products including NS, NNS, SNS, and NSS. By adding excess sulfur, SSNS is also produced.Burning of sulfur and nitrogen doped flames
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane ...
was premixed with fuel in the form of either O2, N2O, or air and burned at ambient pressure. The source of nitrogen was introduced by addition of 1-5 mole% NH3 gas and sulfur by 0.01-0.5 mol% H2S or SF6 gas. A steady state concentration of NS within the flame front is observed by laser-induced fluorescence
Laser-induced fluorescence (LIF) or laser-stimulated fluorescence (LSF) is a spectroscopic method in which an atom or molecule is excited to a higher energy level by the absorption of laser light followed by spontaneous emission of light. It was f ...
(LIF) spectrum.
Flash laser photolysis of tetranitrogen tetrasulfide
N4S4 (g) was obtained by the following reaction: Removal of byproducts leaves only N4S4 in
Removal of byproducts leaves only N4S4 in toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
, which is through a gas inlet pipe into the reaction cell.
Another common method for bulk production of N4S4 production involves the condensation of ammonia with S2Cl2 in inert solvents, however the former method was chosen to avoid isolation of N4S4 in its reactive, solid state.
The NS radical was subsequently identified by LIF spectrum as the product of photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of N4S4 (g) by a 248 nm laser.

Flash and continuous photolysis of Cr(CH3CN)5(NS)2+
Aerated solutions of Cr(CH3CN)5(NS)2+ are highly photoactive and prone to rapid decomposition. Deaerated solutions of Cr(CH3CN)5(NS)2+ inacetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile ( hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
are stable as long as they are kept in the dark. Continuous photolysis using 366 nm light is slow, while using a 355 nm pulsed laser results in faster labilization of NS.
Reactivity
Oligomerization
Evidence suggests that NS can react with itself to reach N2S2, N4S4, and polymers of the form (NS)x. (NS)x forms from polymerization of cyclo-N2S2.
 ''Trans''-NSSN results from direct dimerization of NS.
''Trans''-NSSN results from direct dimerization of NS.
 N3S3 has been observed through photoelectron spectroscopy of vapors of the (SN)x, polymer, but has not yet been characterized further. Attempts to produce N3S3 by oxidation of PNS3N3] were unsuccessful. Its theorized that rapid dimerization to (N3S3)2 will disproportionate irreversibly to N4S4 and N2S2.
N3S3 has been observed through photoelectron spectroscopy of vapors of the (SN)x, polymer, but has not yet been characterized further. Attempts to produce N3S3 by oxidation of PNS3N3] were unsuccessful. Its theorized that rapid dimerization to (N3S3)2 will disproportionate irreversibly to N4S4 and N2S2.

Products of decay with NO₂, NO2
The radical decay time of NS alone is on the order of 1-3 ms. As evident by no change to this decay time upon addition of NO or O2 at ambient temperatures, the NS radical is unreactive with NO and O2. However, rapid, first-order decay is observed with the addition of NO2. This reaction is proposed to proceed through various intermediates, ultimately reaching final products of N2 and SO2. This rapid reaction occurs with arate constant In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction.
For a reaction between reactants A and B to form product C
the reaction rate is often found to have the ...
of k = (2.54 ± 0.12) × 10−11 cm3 molecules−1 s−1 at 295 K. By use of Density Functional Theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
based computational calculations, the minima and transition states of the potential energy surface of this reaction have been predicted.
Astronomical reactivity
Within the inner coma of comets, many reactions are theorized to be relevant to the formation and reactivity of the NS radical.Metal-thionitrosyl complexes
As a ligand, NS acts as a σ-donor and π-acceptor, forming metal-thionitrosyl complexes. Transition-metal thionitrosyl complexes have been prepared by the following procedures: * Sulfur transfer to metal nitrido complexes ** Example: Reflux of (Ph4P) sNCl4and (Ph4P)NCS yields green-brown solid h4Psub>2 s(NS)(NCS)5 * Reaction of trithiazyltrichloride with transition metal complexes ** NSCl3 + OsCl3 > s(NS)Cl3 * Halide abstraction from coordinated thiazyl complexes ** Abstraction of sulfur-bonded fluorine from η5-C5H5)Cr(NO)2(NSF) sF6 by AsF5 > η5-C5H5)Cr(NO)2(NS) sF6sub>2 * Reaction of NS+ salts with transition metal complexes ** NS+SbF6- + (CO)5Br> (CO)5(NS)sup>2+, M=Mn, Re ** NS+AsF6- + η5-C5H5)Fe(CO)2(SO2)sup>+ > η5-C5H5)Fe(CO)2(NS)sF6sub>2 * Reaction of tetrasulfur tetranitride with metal halides or nitrides FromX-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
of many of such metal-thionitrosyl complexes, one can observe that the M-N-S bond angle is nearly linear, suggesting sp hybridization
Hybridization (or hybridisation) may refer to:
*Hybridization (biology), the process of combining different varieties of organisms to create a hybrid
*Orbital hybridization, in chemistry, the mixing of atomic orbitals into new hybrid orbitals
*Nu ...
about N. Short M-N distances and long N-S distances reflect the resonance structure of M=N=S having greater contribution than M-N≡S.
Typical ''v''(NS) IR stretching frequencies are approximately 1065 cm−1 for low-valent transition metal complexes and around 1390 cm−1 in the high valent cases, whereas the free gas-phase radical exhibits a 1204 cm−1 signal.
Electronic structure of Fe(S2CNMe2)2(NS)
The electronic structures of Fe(S2CNMe2)2(NE), where E=O, S, or Se were calculated usingDensity Functional Theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
methods. It was found that the large Mulliken spin density remained concentrated on the Fe(NE) core and Fe-N distances experienced little change from the chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioac ...
atom used. The HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus '' Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely rela ...
of both nitrosyl and thionitrosyl complexes retained 1a1 (dz2) character. The small changes in the energies of the spin orbitals of the complexes, particularly the decreased energetic gap between 2b2 and 1b1 and 2b1 and 1b1 orbitals is attributed to NS being a weaker π-acceptor than NO.
Photoinduced NS transfer from chromium to iron
When a spin-trapping agent, such as Fe(S2CNEt2)2 is present during the photolysis of Cr(CH3CN)5(NS)2+, new S=1/2 EPR bands are observed, attributed to the formation of Fe(S2CNEt2)2(NS), and the signal from Cr(CH3CN)5(NS)2+ disappears. This suggests that the NS radical has transferred from the chromium complex to the iron complex.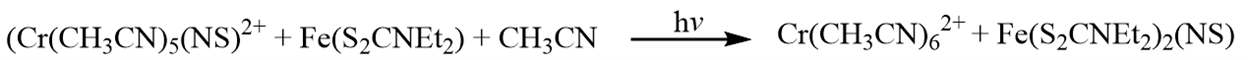 This was particularly significant as it was the first controlled and well-characterized reactivity of NS in solution. Further, it showed the potential for similar reactivity in known reactions with NO, such as use of this iron dithiocarbamate complex.
This was particularly significant as it was the first controlled and well-characterized reactivity of NS in solution. Further, it showed the potential for similar reactivity in known reactions with NO, such as use of this iron dithiocarbamate complex.
Bonding
 The valence electrons of this compound match those of
The valence electrons of this compound match those of nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
. Sulfur mononitride can be described as some average of a set of resonance structures. The singly bonded structure (first resonance structure shown) has little contribution. The formal bond order is considered to be 2.5.


Versus NO
The decreasingelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
with increasingly heavy chalcogenides leads to a reversal of the dipole. In NO, oxygen is the more electronegative element. In NS, nitrogen is more electronegative. The NS radical is significantly more unstable and prone to catenation than NO.
In Astronomy
Molecules in distant astronomical regions can be identified based on their unique rotational transitions, of which the corresponding microwave frequencies are detectable by antennae on Earth. The presence of interstellar sulfur mononitride was first reported in 1975 by back to back letters published in the Astrophysical Journal. Interstellar NS was first identified in the giant molecular cloudSagittarius B2
Sagittarius B2 (Sgr B2) is a giant molecular cloud of gas and dust that is located about from the center of the Milky Way. This complex is the largest molecular cloud in the vicinity of the core and one of the largest in the galaxy, spanning a r ...
(Sgr B2). Its presence was reported in two concurrent articles. Measurements conducted with the National Radia Astronomy Observatory telescope at Kitt Peak, Arizona, picked up millimeter-wavelength radiation in Sgr B2 attributed to ''c-''state transitions of NS in the 2Π1/2 state from J=5/2 to J=3/2 at 115.16 GHz. This assignment was confirmed by measurements conducted at University of Texas Millimeter Wave Observatory on Mount Locke as well, demonstrating J=5/2 to J=3/2 ''c''-state and ''d''-state transitions at 115.16 GHz and 115.6 GHz, respectively. Hyperfine interactions arise from 14N magnetic and electric-quadrupole moments.
NS has been detected in regions responsible for forming massive stars, such as giant molecular clouds like Sg B2 and cold, dark clouds such as L134N and TMC-1. One survey found NS in 12 out of 14 GMC studied, additionally observing the J=7/2 to J=5/2 and J=3/2 to J=1/2 transitions at 161 and 69 GHz, respectively. The abundance of NS in these regions was approximated based on the ratio of observed to intrinsic hyperfine line strengths as well as modeling using a statistical equilibrium program, finding low abundance in all except the Orion molecular cloud.
NS was also observed in the coma of the comets Hyakutake and Hale-Bopp. It’s believed that the observed abundance is higher than gas-phase, ion-molecule models due to an unidentified species X-NS photo-dissociating to release NS.
Industrial Applications
Detection of NS at steady state concentration in the reaction zone of the combustion of methane doped with ammonia and a fuel sulfur such as H2S suggests that NS may be an important reactive intermediate in burning of hydrocarbon flames in a reducing atmosphere, which is relevant to coal pyrolysis and combustion. Fossil fuels contain bound nitrogen, which releases elevated levels ofnitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
emissions during combustion. NOx emissions can be controlled by denitrification of the fuel source, combustion chamber modification, or both. One developing technique is the reburning of NOx, which is reduced to N2. These fuels also contain variable amounts of sulfur, which is oxidized to SO2. Therefore, understanding the reactivity of NO and SO2 is crucial to the process of reburning. The experimental apparatus to test this involved a primary flame for producing combustion products, which were mixed with NO and SO2 to mimic coal burning byproducts. This mixture was fed into the burner at atmospheric pressure. 1-2% decrease in NOx concentration is observed at various percentages of total fuel inlet (reburn ratio) in the presence of 0.1% SO2, which is attributed to the formation of H2S, HS, and the resulting reaction with NO, giving rise to NS. Reaction: HS + NO > NS + OH.
Related compounds
* Trithiazyl trichlorideSee also
*Sulfur nitride Sulfur nitride may refer to a number of sulfur nitrogen compounds:
* pentasulfur hexanitride,
* tetrasulfur tetranitride,
* tetrasulfur dinitride,
* disulfur dinitride,
* polythiazyl,
* thiatetrazole,
Additionally, some unstable species are k ...
References
{{DEFAULTSORT:Mononitrogen Monosulfide Free radicals Inorganic compounds Nitrides Sulfur–nitrogen compounds Diatomic molecules