Metal sulfur dioxide complex on:
[Wikipedia]
[Google]
[Amazon]
In


organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
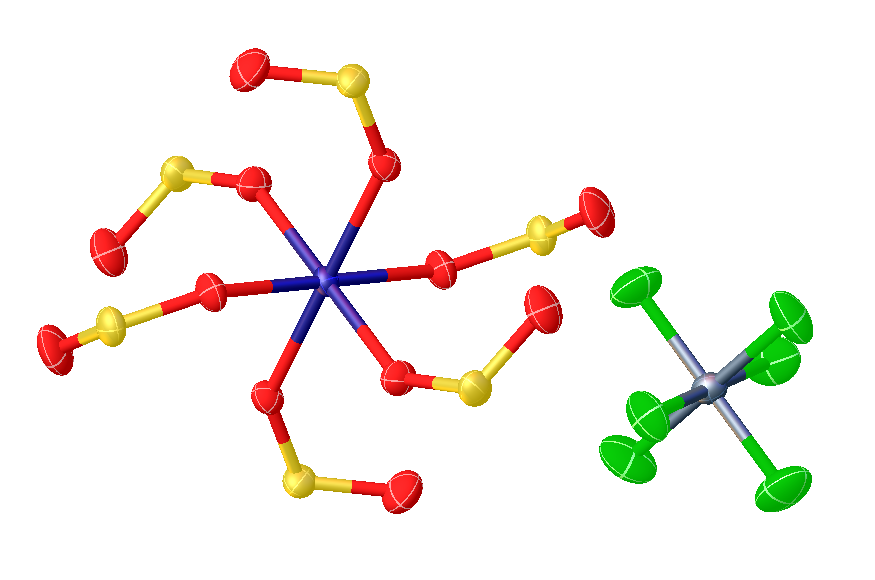
, metal sulfur dioxide complexes are complexes that contain sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
, , bonded to a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
. Such compounds are common but are mainly of theoretical interest. Historically, the study of these compounds has provided insights into the mechanisms of migratory insertion
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanis ...
reactions.
Bonding modes

Sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
forms complexes with many transition metals. Most numerous are complexes with metals i in oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
0 or +1.
In most cases SO2 binds in monodentate fashion, attaching to the metal through sulfur. Such complexes are further subdivided according to the planarity or pyramidalization at sulfur. The various bonding modes are:
* η1-SO2, planar (meaning that the MSO2 subunit forms a plane). In such complexes, SO2 is classified as a 2e donor complemented by pi-back bonding
In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a ''π-acceptor ligand''. It is especially common in the organometallic chemi ...
into the empty pz orbital localized on sulfur.
* η1-SO2, pyramidal (meaning that the MSO2 subunit is pyramidal at sulfur). In such complexes, SO2 is classified as a pure Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
. The structure is similar to that for conventional Lewis base adducts of SO2.
* η2-SO2. Both S and one O centre are attached to the metal. The MSO2 subunit is pyramidal at sulfur. This bonding mode is more common for early metals, which are typically strongly pi-donating.
* η1-SO2, O-bonded. In such cases, SO2 attaches to a metal via one of its two oxygen centres. Such complexes are prevalent for hard metal cations such as Na+ and Al3+. In these compounds the M-O interaction is usually weak.
More exotic bonding modes are known for clusters.
:
Preparation
Complexes of the transition metals are usually generated simply by treating the appropriate metal complex with SO2. Theadduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all co ...
s are often weak. In some cases, SO2 displaces other ligands.
A large number of labile O-bonded SO2 complexes arise from the oxidation of a suspension of the metals in liquid SO2, an excellent solvent.
Reactions
The main reaction of sulfur dioxide promoted by transition metals is its reduction byhydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
. Known as the Claus process
The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
Th ...
, this reaction is conducted on a large scale as a way to remove hydrogen sulfide that arises in hydrotreating processes in refineries.
Insertion of SO2 into metal-ligand bonds
Of academic interest, SO2 acts like aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
towards the alkyl ligand. The pathway for the insertion
Insertion may refer to:
* Insertion (anatomy), the point of a tendon or ligament onto the skeleton or other part of the body
* Insertion (genetics), the addition of DNA into a genetic sequence
*Insertion, several meanings in medicine, see ICD-10-PC ...
of SO2 into metal alkyl bond begins with attack of the alkyl nucleophile on the sulfur centre in SO2. The "insertion" proceed the sulfur dioxide between the metal and the alkyl ligand leads to the ''O'', ''O'-''sulphinate. Alternatively an ''O''-sulphinate can arise. Both of these intermediates commonly convert to an ''S''-sulphinate. ''S''-sulphinate has sulfur–oxygen stretching frequencies from 1250–1000 cm−1 and 1100–1000 cm−1. The ''O'', ''O'-''sulphinate and ''O''-sulphinate are difficult to distinguish as they have stretching frequencies from 1085–1050 cm−1 and 1000–820 cm−1 or lower. The pathway involving the ''O'', ''O' ''sulphinate can generally be ruled out if the original metal complex fulfilled the 18-electron rule because the two metal–oxygen bonds would exceed the 18 electron rule.
The pathway by which SO2 inserts into a square planar alkyl complexes involves the formation of an adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all co ...
. Thereafter, the alkyl ligand migrates to the SO2.

S2O complexes
Several complexes of disulfur monoxide are known. Most are formed by oxidation peroxide oxidation of a disulfur ligand. In these complexes, the ligand is invariably bound in an manner. Selected examples: , , , , , . arises when thedithiocarbamate complex 133px, Tris(dithiocarbamate) complexes have idealized D3 symmetry.
Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are k ...
is oxidized with elemental sulfur in air. Another way to form these complexes is to combine complexes with hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
. Complexes formed in this way are: ; . With hydrosulfide and a base followed by oxygen, can be made.
References