Mercuric sulfide on:
[Wikipedia]
[Google]
[Amazon]
Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the

 HgS is dimorphic with two crystal forms:
* red
HgS is dimorphic with two crystal forms:
* red
 When α-HgS is used as a red pigment, it is known as
When α-HgS is used as a red pigment, it is known as
chemical elements
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler su ...
mercury and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
. It is represented by the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
HgS. It is virtually insoluble in water.
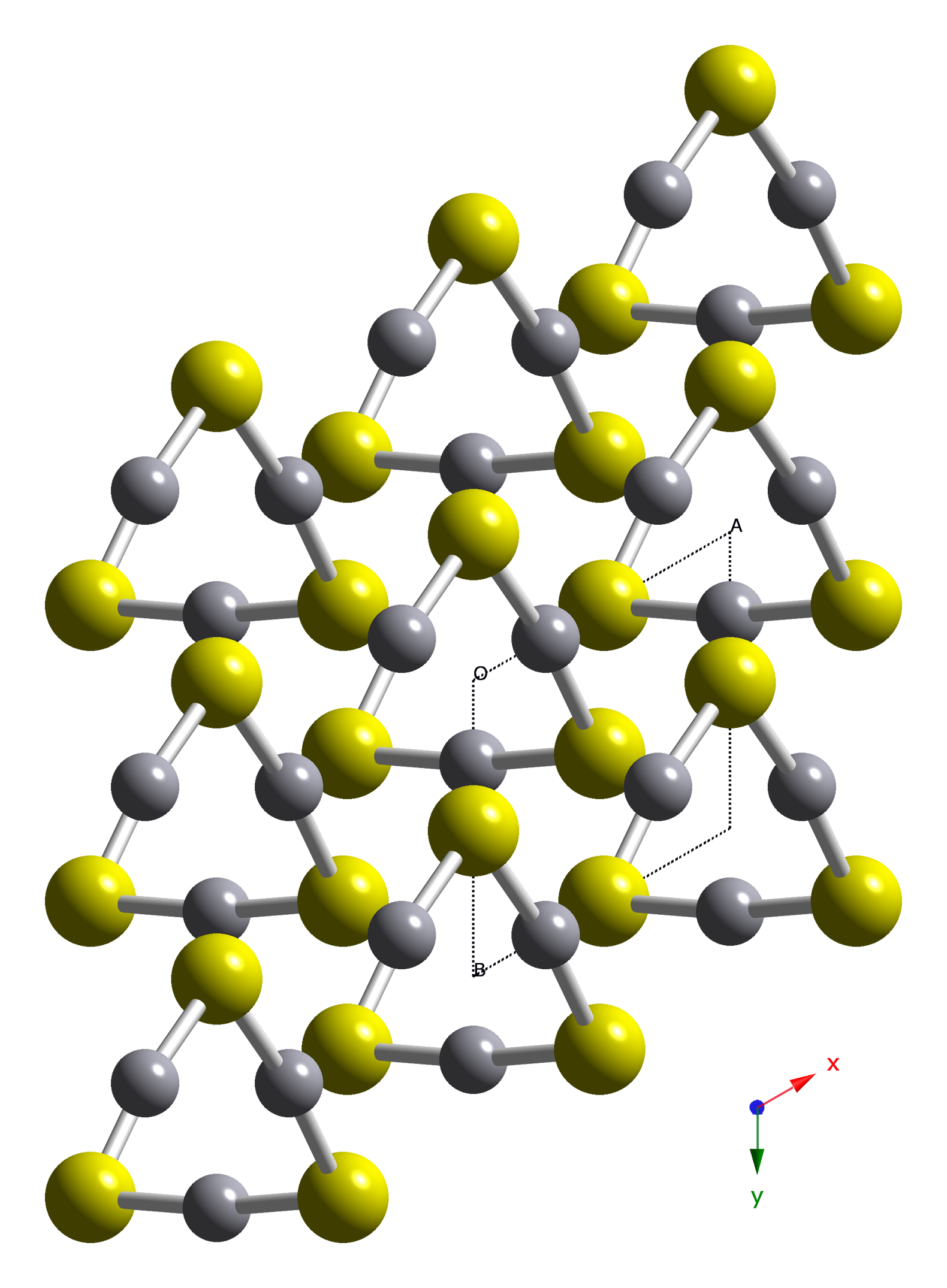
Crystal structure

 HgS is dimorphic with two crystal forms:
* red
HgS is dimorphic with two crystal forms:
* red cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the bri ...
(α-HgS, trigonal
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal ...
, hP6, P3221) is the form in which mercury is most commonly found in nature. Cinnabar has rhombohedral crystal system. Crystals of red are optically active
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
. This is caused by the Hg-S helices in the structure.
* black metacinnabar (β-HgS) is less common in nature and adopts the zinc blende (''T''2d-''F'3m'') crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
.
Preparation and chemistry
β-HgS precipitates as a black solid when Hg(II) salts are treated with H2S. The reaction is conveniently conducted with an acetic acid solution ofmercuric acetate
Mercury(II) acetate is the chemical compound with the formula Hg( O2 CC H3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white water-sol ...
. With gentle heating of the slurry, the black polymorph converts to the red form. β-HgS is unreactive to all but concentrated acids.
Mercury is produced from the cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the bri ...
ore by roasting in air and condensing the vapour.
:HgS → Hg + S
Uses
 When α-HgS is used as a red pigment, it is known as
When α-HgS is used as a red pigment, it is known as vermilion
Vermilion (sometimes vermillion) is a color, color family, and pigment most often made, since antiquity until the 19th century, from the powdered mineral cinnabar (a form of mercury sulfide, which is toxic) and its corresponding color. It i ...
. The tendency of vermilion to darken has been ascribed to conversion from red α-HgS to black β-HgS. However β-HgS was not detected at excavations in Pompeii, where originally red walls darkened, and was attributed to the formation of Hg-Cl compounds (e.g., corderoite
Corderoite is an extremely rare mercury sulfide chloride mineral with formula Hg3S2Cl2. It crystallizes in the isometric crystal system. It is soft, 1.5 to 2 on the Mohs scale, and varies in color from light gray to black and rarely pink or yello ...
, calomel
Calomel is a mercury chloride mineral with formula Hg2Cl2 (see mercury(I) chloride). The name derives from Greek ''kalos'' (beautiful) and ''melas'' (black) because it turns black on reaction with ammonia. This was known to alchemists.
Calomel ...
, and terlinguaite
Terlinguaite is the naturally occurring mineral with formula Hg2 Cl O. It is formed by the weathering of other mercury-containing minerals. It was discovered in 1900 in the Terlingua District of Brewster County
Brewster County is a county l ...
) and calcium sulfate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris ...
, gypsum.
As the mercury cell as used in the chlor-alkali industry (Castner–Kellner process
The Castner–Kellner process is a method of electrolysis on an aqueous alkali chloride solution (usually sodium chloride solution) to produce the corresponding alkali hydroxide, invented by American Hamilton Castner and Austrian Carl Kellner in ...
) is being phased out over concerns over mercury emissions, the metallic mercury from these setups is converted into mercury sulfide for underground storage.
With the band gap of 2.1eV and its stability, it is possible to be used as photo-electrochemical cells
See also
*Mercury poisoning
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashes ...
* Mercury(I) sulfide Mercury(I) sulfide or mercurous sulfide is a hypothetical chemical compound of mercury and sulfur, with elemental formula . Its existence has been disputed; it may be stable below 0 °C or in suitable environments, but is unstable at room te ...
(mercurous sulfide),
References