Mercuric sulfate on:
[Wikipedia]
[Google]
[Amazon]
Mercury(II) sulfate, commonly called mercuric sulfate, is the
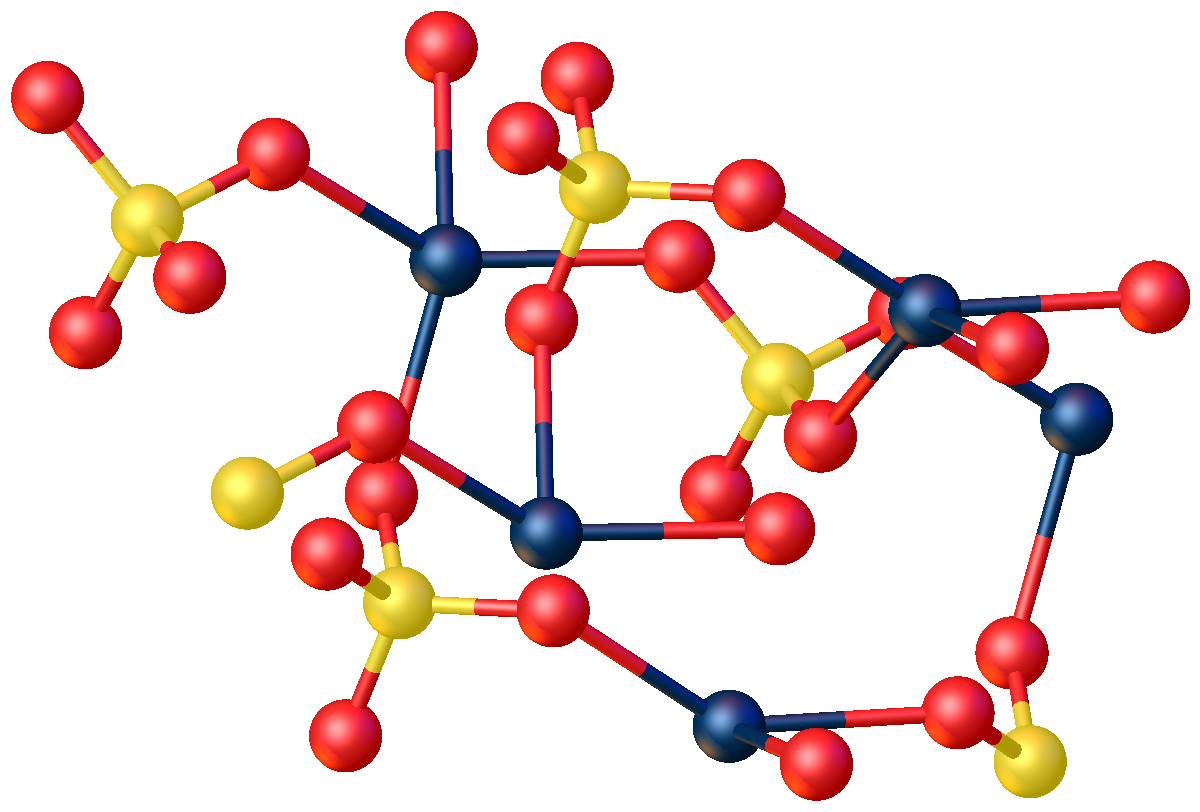 The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å.
The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å.

National Pollutant Inventory – Mercury and compounds Fact Sheet
{{sulfur compounds Sulfates Mercury(II) compounds
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
Hg S O4. It is an odorless solid that forms white granules or crystalline powder. In water, it separates into an insoluble sulfate with a yellow color and sulfuric acid.
Structure
 The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å.
The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å.
History
In 1932, the Japanese chemical companyChisso
The , since 2012 reorganized as JNC (Japan New Chisso), is a Japanese chemical company. It is an important supplier of liquid crystal used for LCDs, but is best known for its role in the 34-year-long pollution of the water supply in Minamata, ...
Corporation began using mercury sulfate as the catalyst for the production of acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
from acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. Though it was unknown at the time, methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It i ...
is formed as side product of this reaction. Exposure and consumption of the mercury waste products, including methylmercury, that were dumped into Minamata Bay by Chisso
The , since 2012 reorganized as JNC (Japan New Chisso), is a Japanese chemical company. It is an important supplier of liquid crystal used for LCDs, but is best known for its role in the 34-year-long pollution of the water supply in Minamata, ...
are believed to be the cause of Minamata disease
Minamata disease is a neurological disease caused by severe mercury poisoning. Signs and symptoms include ataxia, numbness in the hands and feet, general muscle weakness, loss of peripheral vision, and damage to hearing and speech. In extreme ...
in Minamata
is a city located in Kumamoto Prefecture, Japan. It is on the west coast of Kyūshū and faces Amakusa islands. Minamata was established as a village in 1889, re-designated as a town in 1912 and grew into a city in 1949. As of March 2017, the c ...
, Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the n ...
.
Production
Mercury sulfate can be produced by treating mercury with hot concentratedsulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
:
:Hg + 2 H2SO4 → HgSO4 + SO2 + 2 H2O
Alternatively yellow mercuric oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is v ...
reacts also with concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
.
Uses
Denigés' reagent
An acidic solution of mercury sulfate is known asDenigés' reagent
The Denigés' reagent is a reagent used for qualitative analysis. It was developed in 1898 by Georges Denigés (December 25, 1859–February 20, 1951), a French biochemist.
Uses
Denigés' reagent is used to detect isolefin or tertiary alcohol ...
. It was commonly used throughout the 20th century as a qualitative analysis reagent. If Denigés' reagent is added to a solution containing compounds that have tertiary alcohols, a yellow or red precipitate will form.
Production of acetaldehyde
As previously mentioned, Hg S O4 was used as the catalyst for the production ofacetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
from acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
.
Oxymercuration-demercuration of alkenes
Mercury Compounds such as mercury sulfate andmercury(II) acetate
Mercury(II) acetate is the chemical compound with the formula Hg( O2 CC H3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white water-solu ...
are commonly used as catalysts in the oxymercuration-demercuration
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate (AcO–Hg–OAc) in aqueous solution to yield the addition of an ...
, a type of Electrophilic Addition reaction. The hydration of an alkene results in an alcohol that follows regioselectivity that is predicted by Markovnikov's Rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
.
Hydration of alkynes
The reaction scheme is provided below. The conversion of 2,5-dimethyhexyn-2,5-diol to 2,2,5,5-tetramethylte-trahydrofuran using aqueous mercury sulfate without the addition of acid.
Health issues
Inhalation of HgSO4 can result in acute poisoning: causing tightness in the chest, difficulties breathing, coughing and pain. Exposure of HgSO4 to the eyes can cause ulceration of conjunctiva and cornea. If mercury sulfate is exposed to the skin it may cause sensitization dermatitis. Lastly, ingestion of mercury sulfate will cause necrosis, pain, vomiting, and severe purging. Ingestion can result in death within a few hours due to peripheral vascular collapse. It was used in the late 19th century to induce vomiting for medical reasonsReferences
External links
National Pollutant Inventory – Mercury and compounds Fact Sheet
{{sulfur compounds Sulfates Mercury(II) compounds