Marsh test on:
[Wikipedia]
[Google]
[Amazon]
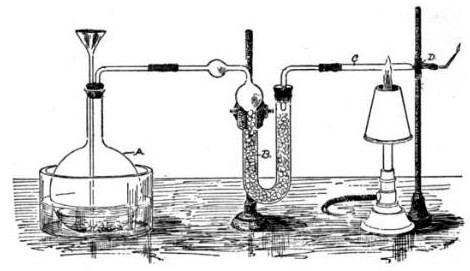 The Marsh test is a highly sensitive method in the detection of
The Marsh test is a highly sensitive method in the detection of
39
Äì57 , url = https://archive.org/details/bub_gb_GyAVfFrjAfwC , quote = james marsh test. , access-date = 2007-12-16 Arsenic Toxicology tests Name reactions
 The Marsh test is a highly sensitive method in the detection of
The Marsh test is a highly sensitive method in the detection of arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, ...
, especially useful in the field of forensic toxicology
Forensic toxicology is the use of toxicology and disciplines such as analytical chemistry, pharmacology and clinical chemistry to aid medical or legal investigation of death, poisoning, and drug use. The primary concern for forensic toxicology is ...
when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836. The method continued to be used, with improvements, in forensic toxicology until the 1970s.
Arsenic, in the form of white arsenic trioxide
Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, whose major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication, it ...
, was a highly favored poison, being odourless, easily incorporated into food and drink, and before the advent of the Marsh test, untraceable in the body. In France, it came to be known as ' ("inheritance powder"). For the untrained, arsenic poisoning
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, bu ...
will have symptoms similar to cholera.
Precursor methods
The first breakthrough in the detection of arsenic poisoning was in 1775 whenCarl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hyd ...
discovered a way to change arsenic trioxide to garlic-smelling arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in ...
gas (AsH3), by treating it with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
(HNO3) and combining it with zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
.
:As2O3 + 6 Zn + 12 HNO3 ‚Üí 2 AsH3 + 6 Zn(NO3)2 + 3 H2O
In 1787, German physician (1739-1805) discovered that if arsenic trioxide were heated in the presence of carbon, the arsenic would sublime. This is the reduction of As2O3 by carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
:
: 2 As2O3 + 3 C ‚Üí 3 CO2 + 4 As
In 1806, Valentin Rose took the stomach of a victim suspected of being poisoned and treated it with potassium carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and gl ...
(K2CO3), calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic ...
(CaO) and nitric acid. Any arsenic present would appear as arsenic trioxide and then could be subjected to Metzger's test.
The most common test (and used even today in water test kits) was discovered by Samuel Hahnemann
Christian Friedrich Samuel Hahnemann (; 10 April 1755 – 2 July 1843) was a German physician, best known for creating the pseudoscientific system of alternative medicine called homeopathy.
Early life
Christian Friedrich Samuel Hahnemann was ...
. It would involve combining a sample fluid with hydrogen sulfide (H2S) in the presence of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
(HCl). A yellow precipitate, arsenic trisulfide
Arsenic trisulfide is the inorganic compound with the formula . It is a dark yellow solid that is insoluble in water. It also occurs as the mineral orpiment (Latin: auripigmentum), which has been used as a pigment called King's yellow. It is produ ...
(As2S3) would be formed if arsenic was present.
Circumstances and methodology
Though precursor tests existed, they had sometimes proven not to be sensitive enough. In 1832, a certain John Bodle was brought to trial for poisoning his grandfather by putting arsenic in his coffee. James Marsh, a chemist working at the Royal Arsenal inWoolwich
Woolwich () is a district in southeast London, England, within the Royal Borough of Greenwich.
The district's location on the River Thames led to its status as an important naval, military and industrial area; a role that was maintained thr ...
, was called by the prosecution to try to detect its presence. He performed the standard test by passing hydrogen sulfide through the suspect fluid. While Marsh was able to detect arsenic, the yellow precipitate did not keep very well, and, by the time it was presented to the jury, it had deteriorated. The jury was not convinced, and John Bodle was acquitted.
Angered and frustrated by this, especially when John Bodle confessed later that he indeed killed his grandfather, Marsh decided to devise a better test to demonstrate the presence of arsenic. Taking Scheele's work as a basis, he constructed a simple glass apparatus capable of not only detecting minute traces of arsenic but also measuring its quantity. Adding a sample of tissue or body fluid to a glass vessel with zinc and acid would produce arsine gas if arsenic was present, in addition to the hydrogen that would be produced regardless by the zinc reacting with the acid. Igniting this gas mixture would oxidize any arsine present into arsenic and water vapor. This would cause a cold ceramic bowl held in the jet of the flame to be stained with a silvery-black deposit of arsenic, physically similar to the result of Metzger's reaction. The intensity of the stain could then be compared to films produced using known amounts of arsenic. Not only could minute amounts of arsenic be detected (as little as 0.02 mg), the test was very specific for arsenic. Although antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
(Sb) could give a false-positive test by forming stibine (SbH3) gas, which decomposes on heating to form a similar black deposit, it would not dissolve in a solution of sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium s ...
(NaOCl), while arsenic would. Bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
(Bi), which also gives a false positive by forming bismuthine (BiH3), similarly can be distinguished by how it resists attack by both NaOCl and ammonium polysulfide (the former attacks As, and the latter attacks Sb).Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001..
Specific reactions involved
The Marsh test treats the sample with sulfuric acid and arsenic-free zinc. Even if there are minute amounts of arsenic present, the zinc reduces thetrivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
arsenic (As3+ ). Here are the two half-reactions:
: Oxidation: Zn ‚Üí Zn2+ + 2 e‚àí
: Reduction: As2O3 + 12 e‚àí + 6 H+ ‚Üí 2 As3‚àí + 3 H2O
Overall, we have this reaction:
: As2O3 + 6 Zn + 6 H+ ‚Üí 2 As3‚àí + 6 Zn2+ + 3 H2O
In an acidic medium, is protonated to form arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in ...
gas (AsH3), so adding sulphuric acid (H2SO4) to each side of the equation we get:
: As2O3 + 6 Zn + 6 H+ + 6 H2SO4 ‚Üí 2 As3‚àí + 6 H2SO4 + 6 Zn2+ + 3 H2O
As the As3‚àí combines with the H+ to form arsine:
: As2O3 + 6 Zn + 6 H+ + 6 H2SO4 ‚Üí 2 AsH3 + 6 ZnSO4 + 3 H2O + 6 H+
By eliminating the common ions:
: As2O3 + 6 Zn + 6 H2SO4 ‚Üí 2 AsH3 + 6 ZnSO4 + 3 H2O
First notable application
Although the Marsh test was efficacious, its first publicly documented use—in fact, the first time evidence fromforensic toxicology
Forensic toxicology is the use of toxicology and disciplines such as analytical chemistry, pharmacology and clinical chemistry to aid medical or legal investigation of death, poisoning, and drug use. The primary concern for forensic toxicology is ...
was ever introduced—was in Tulle
Tulle (; ) is a commune in central France. It is the third-largest town in the former region of Limousin and is the capital of the department of Corrèze, in the region of Nouvelle-Aquitaine. Tulle is also the episcopal see of the Roman Cat ...
, France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of overseas regions and territories in the Americas and the Atlantic, Pacific and Indian Oceans. Its metropolitan area ...
in 1840 with the celebrated Lafarge poisoning case. Charles Lafarge, a foundry owner, was suspected of being poisoned with arsenic by his wife Marie. The circumstantial evidence was great: it was shown that she bought arsenic trioxide from a local chemist, supposedly to kill rats that infested their home. In addition, their maid swore that she had mixed a white powder into his drink. Although the food was found to be positive for the poison using the old methods as well as the Marsh test, when the husband's body was exhumed and tested, the chemists assigned to the case were not able to detect arsenic. Mathieu Orfila, the renowned toxicologist
Toxicology is a scientific discipline, overlapping with biology, chemistry, pharmacology, and medicine, that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnosing and treating expo ...
retained by the defense and an acknowledged authority of the Marsh test, examined the results. He performed the test again, and demonstrated that the Marsh test was not at fault for the misleading results, but, rather, that those who performed it did it incorrectly. Orfila thus proved the presence of arsenic in Lafarge's body using the test. As a result of this, Marie was found guilty and sentenced to life imprisonment.
Effects
The Lafarge case proved to be controversial, for it divided the country into factions who were convinced or otherwise of Mme. Lafarge's guilt; nevertheless, the impact of the Marsh test was great. The French press covered the trial and gave the test the publicity it needed to give the field of forensic toxicology the legitimacy it deserved, although in some ways it trivialized it: actual Marsh test assays were conducted in salons, public lectures and even in some plays that recreated the Lafarge case. The existence of the Marsh test also served a deterrent effect: deliberate arsenic poisonings became rarer because the fear of discovery became more prevalent.In fiction
Marsh test is used in ''Bill Bergson Lives Dangerously
''Bill Bergson Lives Dangerously'' (original Swedish title: ''Mästerdetektiven Blomkvist lever farligt'') is a 1951 Swedish novel written by Astrid Lindgren. It's the 2nd book about the Master Detective Kalle Blomkvist. In this book the Röva ...
'' to prove that a certain chocolate is poisoned with arsenic.
Lord Peter Wimsey
Lord Peter Death Bredon Wimsey (later 17th Duke of Denver) is the fictional protagonist in a series of detective novels and short stories by Dorothy L. Sayers (and their continuation by Jill Paton Walsh). A dilettante who solves mysteries fo ...
’s manservant Bunter uses Marsh’s test in ''Strong Poison
''Strong Poison'' is a 1930 mystery novel by Dorothy L. Sayers, her fifth featuring Lord Peter Wimsey and the first in which Harriet Vane appears.
Plot
The novel opens with mystery author Harriet Vane on trial for the murder of her forme ...
'' to demonstrate that the culprit was secretly in possession of arsenic.
In Alan Bradley's ''As Chimney Sweepers Come To Dust'', 12-year old sleuth and chemistry genius Flavia de Luce uses the Marsh test to determine that arsenic was the murderer's weapon.
In the first episode of the 2017 BBC television series ''Taboo
A taboo or tabu is a social group's ban, prohibition, or avoidance of something (usually an utterance or behavior) based on the group's sense that it is excessively repulsive, sacred, or allowed only for certain persons.''Encyclopædia Britannica ...
'' a mirror test, referencing the Marsh test, is used to verify the protagonist's father was killed via arsenic poisoning. As the setting of the series is between 1814-1820, however, the test's appearance is anachronistic.
In the episode "The King Came Calling" of the first season of ''Ripper Street
''Ripper Street'' is a British mystery drama television series set in Whitechapel in the East End of London starring Matthew Macfadyen, Jerome Flynn, Adam Rothenberg, and MyAnna Buring. It begins in 1889, six months after the infamous Jack the ...
'', police surgeon Homer Jackson (Matthew Rothenberg Matthew Rothenberg (born February 16, 1965) is an American journalist and co-author of "You're Better Than Your Job Search" with TheLadders.com CEO Marc Cenedella.
Rothenberg, the son of American poet Jerome Rothenberg, worked at ''MacWEEK'' in Sa ...
) performs Marsh's test on the contents of a poisoning victim and determines that the fatal poison was antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
, not arsenic, since the chemical residue deposited by the flames does not dissolve in sodium hypochlorite.
See also
* James Marsh * Nascent hydrogen * Devarda's alloy *Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in ...
* Stibine
* Bismuthine
References
External links
* * {{cite book , last = Wanklyn , first = James Alfred , title = Arsenic , publisher = Kegan Paul, Trench, Trübner & Co. Ltd. , year = 1901 , location = London , pages39
Äì57 , url = https://archive.org/details/bub_gb_GyAVfFrjAfwC , quote = james marsh test. , access-date = 2007-12-16 Arsenic Toxicology tests Name reactions