Krypton on:
[Wikipedia]
[Google]
[Amazon]
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a
 Krypton was discovered in
Krypton was discovered in
Kr + F2 -> KrF2
Krypton gas in a krypton fluoride laser absorbs energy from a source, causing the krypton to react with fluorine gas, producing the exciplex krypton fluoride, a temporary 2Kr + F2 -> 2KrF
The complex can undergo spontaneous or stimulated emission, reducing its energy state to a metastable, but highly repulsive ground state. The ground state complex quickly dissociates into unbound atoms:
:2KrF -> 2Kr + F2
The result is an exciplex laser which radiates energy at 248 nm, near the 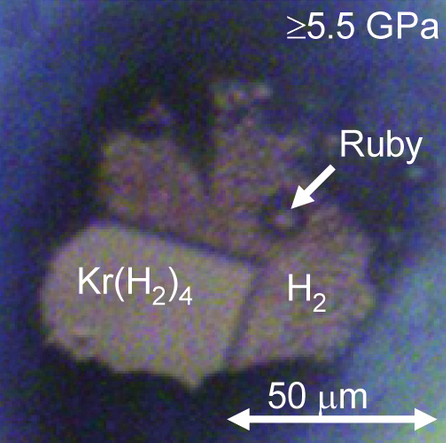
 Compounds with krypton bonded to atoms other than fluorine have also been discovered. There are also unverified reports of a barium
Compounds with krypton bonded to atoms other than fluorine have also been discovered. There are also unverified reports of a barium
 Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed
Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed
 Krypton is considered to be a non-toxic asphyxiant.
Krypton is considered to be a non-toxic asphyxiant.Properties of Krypton
. Pt.chemicalstore.com. Retrieved on 2015-11-30. Being
"Krypton 85: a Review of the Literature and an Analysis of Radiation Hazards"
Environmental Protection Agency, Office of Research and Monitoring, Washington (1972)
at ''
Krypton Fluoride Lasers
Plasma Physics Division Naval Research Laboratory {{Authority control Chemical elements Noble gases
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Kr and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
36. It is a colorless, odorless, tasteless noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet ligh ...
s. With rare exceptions, krypton is chemically inert.
Krypton, like the other noble gases, is used in lighting and photography
Photography is the art, application, and practice of creating durable images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is employe ...
. Krypton light has many spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to iden ...
s, and krypton plasma is useful in bright, high-powered gas lasers (krypton ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
and excimer
An excimer (originally short for excited dimer) is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which has a valence shell completely filled with electrons (for example, noble gases). In this case, form ...
lasers), each of which resonates and amplifies a single spectral line. Krypton fluoride also makes a useful laser medium
The active laser medium (also called gain medium or lasing medium) is the source of optical gain within a laser. The gain results from the stimulated emission of photons through electronic or molecular transitions to a lower energy state from a h ...
. From 1960 to 1983, the official definition of meter was based on the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
of one spectral line of krypton-86, because of the high power and relative ease of operation of krypton discharge tube
A gas-filled tube, also commonly known as a discharge tube or formerly as a Plücker tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric ...
s.
History
 Krypton was discovered in
Krypton was discovered in Britain
Britain most often refers to:
* The United Kingdom, a sovereign state in Europe comprising the island of Great Britain, the north-eastern part of the island of Ireland and many smaller islands
* Great Britain, the largest island in the United King ...
in 1898 by William Ramsay
Sir William Ramsay (; 2 October 1852 – 23 July 1916) was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous element ...
, a Scottish chemist, and Morris Travers
Morris William Travers, FRS (24 January 1872 – 25 August 1961) was an English chemist who worked with Sir William Ramsay in the discovery of xenon, neon and krypton. His work on several of the rare gases earned him the name ''Rare gas T ...
, an English chemist, in residue left from evaporating nearly all components of liquid air. Neon was discovered by a similar procedure by the same workers just a few weeks later. William Ramsay was awarded the 1904 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
for discovery of a series of noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
es, including krypton.
In 1960, the International Bureau of Weights and Measures defined the meter as 1,650,763.73 wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
s of light emitted in the vacuum corresponding to the transition between the 2p10 and 5d5 levels in the isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
krypton-86. This agreement replaced the 1889 international prototype meter, which was a metal bar located in Sèvres
Sèvres (, ) is a commune in the southwestern suburbs of Paris, France. It is located from the centre of Paris, in the Hauts-de-Seine department, Île-de-France region. The commune, which had a population of 23,251 as of 2018, is known for ...
. This also obsoleted the 1927 definition of the ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
based on the red cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
spectral line, replacing it with 1 Å = 10−10 m. The krypton-86 definition lasted until the October 1983 conference, which redefined the meter as the distance that light travels in vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often di ...
during 1/299,792,458 s.
Characteristics
Krypton is characterized by several sharp emission lines (spectral signature
Spectral signature is the variation of reflectance or emittance of a material with respect to wavelengths (i.e., reflectance/emittance as a function of wavelength). The spectral signature of stars indicates the composition of the stellar atmosph ...
s) the strongest being green and yellow. Krypton is one of the products of uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
fission. Solid krypton is white and has a face-centered cubic crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns ...
, which is a common property of all noble gases (except helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, which has a hexagonal close-packed crystal structure).
Isotopes
Naturally occurring krypton in Earth's atmosphere is composed of five stableisotopes
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
, plus one isotope (78Kr) with such a long half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
(9.2×1021 years) that it can be considered stable. (This isotope has the second-longest known half-life among all isotopes for which decay has been observed; it undergoes double electron capture to 78 Se). In addition, about thirty unstable isotopes and isomers
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
...
are known. Traces of 81Kr, a cosmogenic nuclide
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom ...
produced by the cosmic ray
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own ...
irradiation of 80Kr, also occur in nature: this isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
is radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
with a half-life of 230,000 years. Krypton is highly volatile and does not stay in solution in near-surface water, but 81Kr has been used for dating
Dating is a stage of romantic relationships in which two individuals engage in an activity together, most often with the intention of evaluating each other's suitability as a partner in a future intimate relationship. It falls into the categor ...
old (50,000–800,000 years) groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidated ...
.
85Kr is an inert radioactive noble gas with a half-life of 10.76 years. It is produced by the fission of uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
, such as in nuclear bomb testing and nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s. 85Kr is released during the reprocessing of fuel rod
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
s from nuclear reactors. Concentrations at the North Pole
The North Pole, also known as the Geographic North Pole or Terrestrial North Pole, is the point in the Northern Hemisphere where the Earth's rotation, Earth's axis of rotation meets its surface. It is called the True North Pole to distingu ...
are 30% higher than at the South Pole
The South Pole, also known as the Geographic South Pole, Terrestrial South Pole or 90th Parallel South, is one of the two points where Earth's axis of rotation intersects its surface. It is the southernmost point on Earth and lies antipod ...
due to convective mixing.
Chemistry
Like the other noble gases, krypton is chemically highly unreactive. The rather restricted chemistry of krypton in the +2 oxidation state parallels that of the neighboring elementbromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
in the +1 oxidation state; due to the scandide contraction it is difficult to oxidize the 4p elements to their group oxidation states. Until the 1960s no noble gas compounds had been synthesized.
Following the first successful synthesis of xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
compounds in 1962, synthesis of krypton difluoride
Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances ...
() was reported in 1963. In the same year, was reported by Grosse, ''et al.'', but was subsequently shown to be a mistaken identification. Under extreme conditions, krypton reacts with fluorine to form KrF2 according to the following equation:
:complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
in an excited energy state:
:ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
portion of the spectrum
A spectrum (plural ''spectra'' or ''spectrums'') is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors ...
, corresponding with the energy difference between the ground state and the excited state of the complex.

 Compounds with krypton bonded to atoms other than fluorine have also been discovered. There are also unverified reports of a barium
Compounds with krypton bonded to atoms other than fluorine have also been discovered. There are also unverified reports of a barium salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
of a krypton oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce ...
. ArKr+ and Kr H+ polyatomic ions have been investigated and there is evidence for Kr Xe or KrXe+.
The reaction of with produces an unstable compound, , that contains a krypton-oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
bond. A krypton-nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
bond is found in the cation C≡N–Kr–F produced by the reaction of with C≡NHAsF] below −50 °C. HKrCN and HKrC≡CH (krypton hydride-cyanide and hydrokryptoacetylene) were reported to be stable up to 40 kelvin, K.
Krypton hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
(Kr(H2)4) crystals can be grown at pressures above 5 GPa. They have a face-centered cubic structure where krypton octahedra are surrounded by randomly oriented hydrogen molecules.
Natural occurrence
Earth has retained all of the noble gases that were present at its formation excepthelium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
. Krypton's concentration in the atmosphere is about 1 ppm. It can be extracted from liquid air by fractional distillation. The amount of krypton in space is uncertain, because measurement is derived from meteoric activity and solar winds. The first measurements suggest an abundance of krypton in space.
Applications
 Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed
Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed photography
Photography is the art, application, and practice of creating durable images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is employe ...
. Krypton gas is also combined with mercury to make luminous signs that glow with a bright greenish-blue light.
Krypton is mixed with argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
in energy efficient fluorescent lamps, reducing the power consumption, but also reducing the light output and raising the cost. Krypton costs about 100 times as much as argon. Krypton (along with xenon) is also used to fill incandescent lamps to reduce filament evaporation and allow higher operating temperatures. A brighter light results with more blue color than conventional incandescent lamps.
Krypton's white discharge is sometimes used as an artistic effect in gas discharge "neon" tubes. Krypton produces much higher light power than neon in the red spectral line region, and for this reason, red lasers for high-power laser light-shows are often krypton lasers with mirrors that select the red spectral line for laser amplification and emission, rather than the more familiar helium-neon variety, which could not achieve the same multi-watt outputs.
The krypton fluoride laser is important in nuclear fusion energy research in confinement experiments. The laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The fi ...
has high beam uniformity, short wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
, and the spot size can be varied to track an imploding pellet.
In experimental particle physics
Particle physics or high energy physics is the study of fundamental particles and forces that constitute matter and radiation. The fundamental particles in the universe are classified in the Standard Model as fermions (matter particles) an ...
, liquid krypton is used to construct quasi-homogeneous electromagnetic calorimeters. A notable example is the calorimeter of the NA48 experiment at CERN containing about 27 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s of liquid krypton. This usage is rare, since liquid argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
is less expensive. The advantage of krypton is a smaller Molière radius of 4.7 cm, which provides excellent spatial resolution with little overlapping. The other parameters relevant for calorimetry are: radiation length In physics, the radiation length is a characteristic of a material, related to the energy loss of high energy particles electromagnetically interacting with it.
Definition
In materials of high atomic number (e.g. W, U, Pu) the electrons of energie ...
of X0=4.7 cm, and density of 2.4 g/cm3.
The sealed spark gap assemblies in ignition exciters in some older jet engines contain a small amount of krypton-85 to produce consistent ionization levels and uniform operation.
Krypton-83 has application in magnetic resonance imaging (MRI) for imaging airways. In particular, it enables the radiologist to distinguish between hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
and hydrophilic surfaces containing an airway.
Although xenon has potential for use in computed tomography (CT) to assess regional ventilation, its anesthetic properties limit its fraction in the breathing gas to 35%. A breathing mixture of 30% xenon and 30% krypton is comparable in effectiveness for CT to a 40% xenon fraction, while avoiding the unwanted effects of a high partial pressure of xenon gas.
The metastable isotope
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ha ...
krypton-81m is used in nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
for lung ventilation/perfusion scan
A ventilation/perfusion lung scan, also called a V/Q lung scan, or ventilation/perfusion scintigraphy, is a type of medical imaging using scintigraphy and medical isotopes to evaluate the circulation of air and blood within a patient's lungs, in ...
s, where it is inhaled and imaged with a gamma camera
A gamma camera (γ-camera), also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy. The applications of scintigraphy include early drug development ...
.
Krypton-85 in the atmosphere has been used to detect clandestine nuclear fuel reprocessing facilities in North Korea
North Korea, officially the Democratic People's Republic of Korea (DPRK), is a country in East Asia. It constitutes the northern half of the Korean Peninsula and shares borders with China and Russia to the north, at the Yalu (Amnok) and T ...
and Pakistan
Pakistan ( ur, ), officially the Islamic Republic of Pakistan ( ur, , label=none), is a country in South Asia. It is the world's fifth-most populous country, with a population of almost 243 million people, and has the world's second-lar ...
. Those facilities were detected in the early 2000s and were believed to be producing weapons-grade plutonium. Krypton-85 is a medium lived fission product and thus escapes from spent fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor a ...
when the cladding is removed. This release is usually not dangerous as krypton is chemically inert and disperses widely in the atmosphere but it can be detected by sufficiently sensitive equipment.
Krypton is used occasionally as an insulating gas between window panes.
SpaceX Starlink
Starlink is a satellite internet constellation operated by SpaceX, providing satellite Internet access coverage to 45 countries. It also aims for global mobile phone service after 2023. SpaceX started launching Starlink satellites in 2019. As ...
use krypton as propellant for their electric propulsion system.
Precautions
 Krypton is considered to be a non-toxic asphyxiant.
Krypton is considered to be a non-toxic asphyxiant.. Pt.chemicalstore.com. Retrieved on 2015-11-30. Being
lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipo ...
, Krypton has a significant anaestethic effect (although the mechanism of this phenomenon is still not fully clear, there's good evidence that the two properties are mechanistically related), with narcotic potency seven times greater than air, and breathing an atmosphere of 50% krypton and 50% natural air (as might happen in the locality of a leak) causes narcosis in humans similar to breathing air at four times atmospheric pressure. This is comparable to scuba diving at a depth of and could affect anyone breathing it. At the same time, that mixture would contain only 10% oxygen (rather than the normal 20%) and hypoxia would be a greater concern.
References
Further reading
* William P. Kir"Krypton 85: a Review of the Literature and an Analysis of Radiation Hazards"
Environmental Protection Agency, Office of Research and Monitoring, Washington (1972)
External links
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Krypton Fluoride Lasers
Plasma Physics Division Naval Research Laboratory {{Authority control Chemical elements Noble gases