Josiphos ligands on:
[Wikipedia]
[Google]
[Amazon]
A Josiphos ligand is a type of 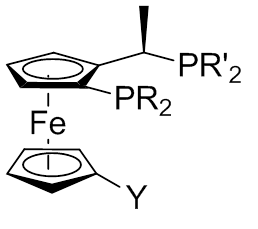
chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
diphosphine which has been modified to be substrate-specific; they are widely used for enantioselective synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecu ...
. -U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer and A. Togni, Top. Catal ., 2002, 19, 3. They are named after the technician who made the first one, Josi Puleo.

Applications
Homogeneous catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysi ...
is often used for enantioselective transformations. The ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
carry chiral information and thus they are modified for individual substrates. Ligands can also influence the chemoselectivity
Chemoselectivity is the preferential outcome of a chemical reaction over a set of possible alternative reactions.
In another definition, chemoselectivity refers to the selective reactivity of one functional group in the presence of others; often ...
of the catalyst. The Josiphos ligands, often called privileged ligands, are important because of their ability to give high yields in enantioselective synthesis.
Josiphos ligands were developed in the 1990s by Antonio Togni in studies on ferrocenyl ligands previously discovered by T. Hayashi (1986). These studies focused of an Au(I)-catalyzed aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two ...
at The Central Research Laboratories of the former Ciba (now Novartis
Novartis AG is a Swiss-American multinational pharmaceutical corporation based in Basel, Switzerland and
Cambridge, Massachusetts, United States (global research).name="novartis.com">https://www.novartis.com/research-development/research-lo ...
). Diphosphine ligands were prepared with secondary phosphines, they are today known as Josiphos ligands family, which gets the name after Josi Puleo, the technician who prepared the first one. It was first tried in an Ru-catalyzed enamide hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
synthesis resulting in ee’s higher than 99% and TOF of 1000h−1. The ligand was applied to the synthesis of the herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
(S)-metolachlor, the active ingredient in the most common herbicide in the United States. The synthesis proceeds via the enantioselective hydrogenation of an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
(figure 2). The reaction proceeds with 100% conversion with TON
Ton is the name of any one of several units of measure. It has a long history and has acquired several meanings and uses.
Mainly it describes units of weight. Confusion can arise because ''ton'' can mean
* the long ton, which is 2,240 pounds
...
over 7,000,000 and Turnover frequency (''TOF'') higher than 2,000,000 h−1. This process is the largest-scale application of enantioselective hydrogenation, producing over 10,000 tons/year of the desired product with 79% ee.
Figure 2: Xyliphos ligand
The ligands are also used in non-enantioselective reactions. They have been good ligands in Pd-catalyzed reaction of aryl chloride In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhi ...
s and aryl vinyl tosylates with TON of 20,000 or higher. Also in the Pd/Josiphos catalyzed carbonylation. Coupling with Grignards and Negishi coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) specie ...
reactions
A variety of Josiphos ligands are commercially available, under licence from Solvias. The (R-S) and its enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
are commonly used because they provide higher yields and higher enantioselectivities than the diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
(R,R). The ferrocene scaffold has proved to be versatile.Zhou Q.L, (2011). Privileged Chiral Ligands and Catalyst. pp.93-127 One structural parameter that influences reactivity is the bite angle. The P1-M-P2 angle has an average value of 92.7°.
Figure 3: View of general conformation of a Josiphos ligands complex
The general consensus for the naming is abbreviating the individual ligand as (R)-(S)-R2PF-PR’2. The substituent on the Cp is written in front of the F and the R on the chiral center after the F.
Synthesis of Josiphos ligands
The preparation of Josiphos ligands starts fromUgi's amine
Ugi’s amine is a chemical compound named for the chemist who first reported its synthesis in 1970, Ivar Karl Ugi, Ivar Ugi. It is a ferrocene derivative (chemistry), derivative. Since its first report, Ugi’s amine has found extensive use as t ...
.
Figure 4: Scheme for general synthesis of the Josiphos ligands
An important improvement since the first intent, and already pointed out in figure 4, has been using the N(CH3)2 group as the leaving group and not acetate. Also found was that the use of acetic acid as solvent gave better yields.
Reactions using Josiphos ligands
Some reactions that are accomplished using M-Josiphos complexes as catalyst are listed below. 1) Hydroboration of styrene Figure 5: Hydroboration of styrene With ee’s up to 92% and TOF of 5-10h−1. The reaction is conducted at -78 °C. Hayashi’s Rh-binap complex gives better yield. 2) Hydroformylation of Styrene Figure 6: Hydroformylation of Styrene Yields of up to 78 %ee of the (R) product, however low TON and TOF, 10-210 and 1-14h−1, respectively. 3) Reductive amination Figure 7: Reductive amination This is the preparation of (S)-metolachlor. It is highly dependent on the solvent where AcOH is necessary to achieve good yields and a 100% conversion. 4) Hydrogenation of exocyclic methyl imine Figure 8:Hydrogenation of exocyclic methyl imine This reaction is the key step to for the synthesis of a HIV integrase inhibitor, Crixivan. This reaction gave 97% ee with TON and TOF of 1000 and 480h−1, respectively. This is one of the few reactions known of a homogeneous heteroarene hydrogenation. Bulky R groups increase the catalyst’s performance. 5) Asymmetric synthesis of chromanoylpyridine derivatives Figure 9: Asymmetric synthesis of chromanoylpyridine derivatives . Broger, Y. Crameri and P. Jones, WO 99/01 453. (1997), assigned to Hoffman-La Roche This reaction shows an intermediate for the synthesis of a chromanoylpyridine derivative used for hair growth and as an antihypertensive agent. This reaction occurs with high enantioselectivity, but low activity. Other reactions where Josiphos ligands can be used are; hydrogenation of CC bonds, hydrogenation of CN, CC and CO, catalyzed allylic substitution, hydrocarboxylation, Michael addition, allylic alkylation, Heck reaction, ring-opening of oxabicycles, isomerization of allylamines and allylic substitution.References
{{reflist Coordination chemistry Phosphines Ferrocenes Ligands Diphosphines