Jacobsen epoxidation on:
[Wikipedia]
[Google]
[Amazon]

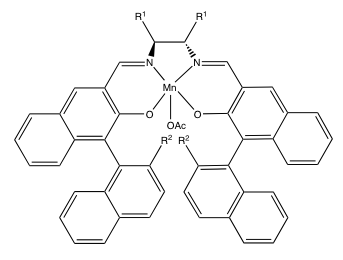 The Jacobsen
The Jacobsen 
 The radical intermediate accounts for the formation of mixed epoxides when conjugated dienes are used as substrates.
:
The radical intermediate accounts for the formation of mixed epoxides when conjugated dienes are used as substrates.
:
 The Jacobsen
The Jacobsen epoxidation
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
which allows enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is ''tert''-butyl hydroperoxide. The method relies on a catalyst formed fro ...
(used to form epoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
from the double bond in allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
ic alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
). The Jacobsen epoxidation gains its stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
from a ''C2'' symmetric
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definiti ...
manganese(III) salen-like ligand, which is used in catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
amounts. The manganese atom transfers an oxygen atom from chlorine bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
or similar oxidant. The reaction takes its name from its inventor, Eric Jacobsen, with Tsutomu Katsuki
Tsutomu Katsuki (September 23, 1946 – October 13, 2014) was an organic chemist who primarily focused on asymmetric oxidation reactions utilizing transition metal catalysts.
Education
Katsuki performed doctoral studies in the lab of Masaru Ya ...
sometimes being included. Chiral-directing catalysts are useful to organic chemists trying to control the stereochemistry of biologically active compounds and develop enantiopure drug An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules (proteins, sugars, etc.) are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind di ...
s.
Several improved procedures have been developed.
A general reaction scheme follows:
:
History
In the early 1990s, Jacobsen and Katsuki independently released their initial findings about their catalysts for the enantioselective epoxidation of isolated alkenes. In 1991, Jacobsen published work where he attempted to perfect the catalyst. He was able to obtain ee values above 90% for a variety of ligands. Also, the amount of catalyst used was no more than 15% of the amount of alkene used in the reaction.General features
The degree of enantioselectivity depends on numerous factors, namely the structure of the alkene, the nature of the axial donor ligand on the active oxomanganese species and the reaction temperature. Cyclic and acyclic ''cis''-1,2-disubstituted alkenes are epoxidized with almost 100% enantioselectivity whereas ''trans''-1,2-disubstituted alkenes are poor substrates for Jacobsen's catalysts but yet give higher enantioselectivities when Katsuki's catalysts are used. Furthermore, the enantioselective epoxidation of conjugated dienes is much higher than that of the nonconjugated dienes. The enantioselectivity is explained by either a "top-on" approach (Jacobsen) or by a "side-on" approach (Katsuki) of the alkene.Mechanism
The mechanism of the Jacobsen–Katsuki epoxidation is not fully understood, but most likely a manganese(V)-species (similar to theferryl
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligan ...
intermediate of Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
) is the reactive intermediate which is formed upon the oxidation of the Mn(III)-salen complex A metal salen complex is a coordination compound between a metal cation and a ligand derived from ''N'',''N''′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt , usua ...
. There are three major pathways. The concerted pathway, the metalla oxetane pathway and the radical pathway. The most accepted mechanism is the concerted pathway mechanism. After the formation of the Mn(V) complex, the catalyst is activated and therefore can form epoxides with alkenes. The alkene comes in from the "top-on" approach (above the plane of the catalyst) and the oxygen atom now is bonded to the two carbon atoms (previously C=C bond) and is still bonded to the manganese metal. Then, the Mn–O bond breaks and the epoxide is formed. The Mn(III)-salen complex is regenerated, which can then be oxidized again to form the Mn(V) complex.
: The radical intermediate accounts for the formation of mixed epoxides when conjugated dienes are used as substrates.
:
The radical intermediate accounts for the formation of mixed epoxides when conjugated dienes are used as substrates.
:
References
{{Reflist Epoxidation reactions Organic oxidation reactions Name reactions