Isopentenyl-diphosphate delta isomerase on:
[Wikipedia]
[Google]
[Amazon]
Isopentenyl pyrophosphate isomerase (, IPP isomerase), also known as Isopentenyl-diphosphate delta isomerase, is an
isomerase
Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:
A–B ↠...
that catalyzes the conversion of the relatively un-reactive isopentenyl pyrophosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of i ...
(IPP) to the more-reactive electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ...
(DMAPP). This isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
is a key step in the biosynthesis of isoprenoids through the mevalonate pathway
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl ...
and the MEP pathway.
:isopentenyl diphosphate dimethylallyl diphosphate
This enzyme belongs to the family of isomerase
Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:
A–B ↠...
s, specifically those intramolecular oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually u ...
s transposing C=C bonds. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivial ...
of this enzyme class is isopentenyl-diphosphate Delta3-Delta2-isomerase. Other names in common use include isopentenylpyrophosphate Delta-isomerase, methylbutenylpyrophosphate isomerase, and isopentenylpyrophosphate isomerase.
Enzyme mechanism
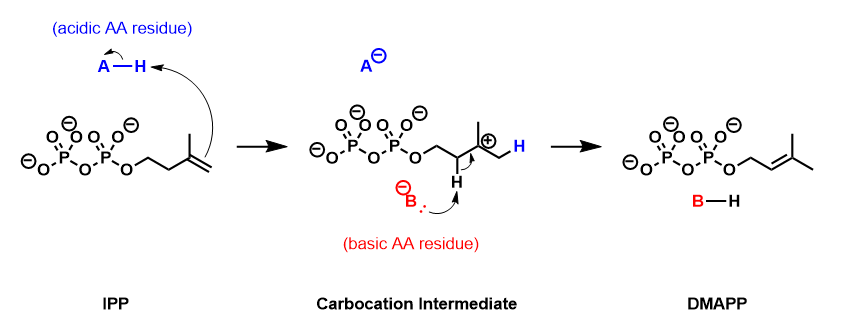
IPP isomerase catalyzes the isomerization of IPP to DMAPP by anantarafacial Antarafacial ( Woodward-Hoffmann symbol a) and suprafacial (s) are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction cent ...
transposition of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
. The empirical evidence suggests that this reaction proceeds by a protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
/deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
mechanism, with the addition of a proton to the ''re''-face of the inactivated C3-C4 double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
resulting in a transient carbocation intermediate. The removal of the pro-R proton from C2 forms the C2-C3 double bond of DMAPP.

Enzyme structure
Crystallographic
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics ( condensed matter physics). The w ...
studies have observed that the active form of IPP isomerase is a monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
with alternating α-helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
and β-sheets. The active site of IPP isomerase is deeply buried within the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
and consists of a glutamic acid residue and a cysteine residue that interact with opposite sides of the IPP Substrate (biochemistry), substrate, consistent with the antarafacial stereochemistry of isomerization. The origin of the initial protonation step has not been conclusively established. Recent evidence suggests that the glutamic acid residue is involved in the protonating step despite the observation that its carboxylic acid Side chain, side-chain is stabilized in its carboxylate form. This discrepancy has been addressed by the discovery of a water molecule in the active site of human IPP isomerase, suggesting a mechanism where the glutamine residue intermolecular force, polarizes the double bond of IPP and makes it more susceptible to protonation by water.
IPP isomerase also requires a divalent cation to fold into its active conformation. The enzyme contains several amino acids, including the catalytic glutamate, that are involved in dipolar bond, coordinating with magnesium, Mg2+ or Manganese, Mn2+. The coordination of the metal cation to the glutamate residue stabilizes the carbiocation intermediate after protonation.
Structural studies
As of late 2007, 25 tertiary structure, structures have been solved for this class of enzymes, with Protein Data Bank, PDB accession codes , , , , , , , , , , , , , , , , , , , , , , , , and .Biological function
The protonation of an inactivated double bond is rarely seen in nature, highlighting the unique catalytic mechanism of IPP isomerase. The isomerization of IPP to DMAPP is a crucial step in the synthesis of isoprenoids and isoprenoid-derivatives, compounds that play vital roles in the biosynthetic pathways of all living organisms. Because of the importance of the melavonate pathway in isoprenoid biosynthesis, IPP isomerase is found in a variety of different cellular compartments, including plastids and mammalian mitochondria.Disease relevance
Mutations in ''IDI1'', the gene that codes for IPP isomerase 1, have been implicated in decreased viability in a number of organisms, including the yeast ''Saccharomyces cerevisiae'', the nematode ''Caenorhabditis elegans'' and the plant ''Arabidopsis thaliana''. While there have been no evidence directly implicating ''IDI1'' mutations in human disease, full genome sequencing, genomic analysis has identified a copy-number variation, copy-number gain near two IPP isomerase genes in a substantial proportion of patients with sporadic amyotrophic lateral sclerosis, suggesting that the isomerase may play a role in this disease.References
External links
* {{Mevalonate pathway EC 5.3.3 Enzymes of known structure