Integrated risk information system on:
[Wikipedia]
[Google]
[Amazon]
 The Integrated Risk Information System (IRIS) is an environmental assessment program operated by the
The Integrated Risk Information System (IRIS) is an environmental assessment program operated by the
Integrated Risk Information System
- EPA
Office of Management and Budget; Information and Regulatory affairs
Environmental toxicology Hazard analysis Risk analysis United States Environmental Protection Agency {{Environment-stub
 The Integrated Risk Information System (IRIS) is an environmental assessment program operated by the
The Integrated Risk Information System (IRIS) is an environmental assessment program operated by the U.S. Environmental Protection Agency
The Environmental Protection Agency (EPA) is an Independent agencies of the United States government, independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon pro ...
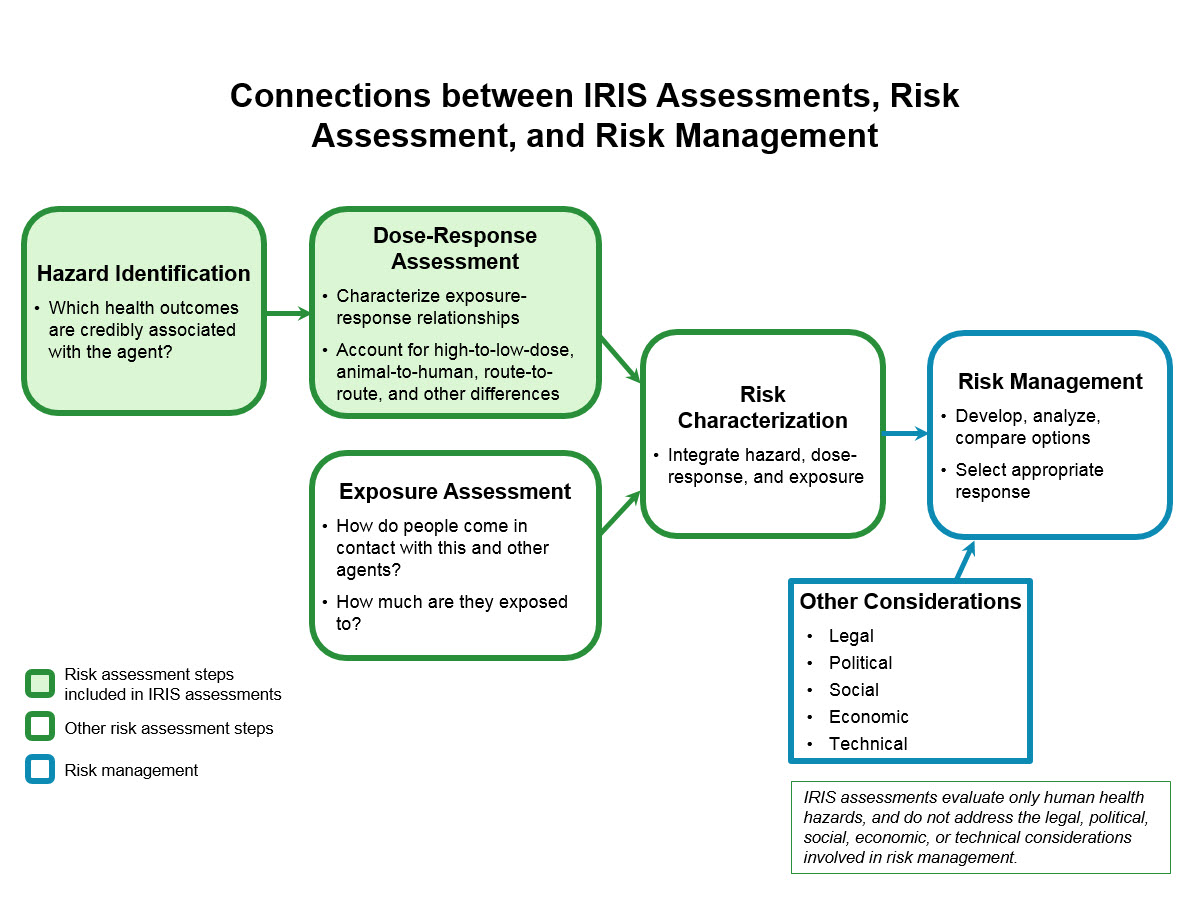
(EPA). The IRIS program is focused on risk assessment
Broadly speaking, a risk assessment is the combined effort of:
# identifying and analyzing potential (future) events that may negatively impact individuals, assets, and/or the environment (i.e. hazard analysis); and
# making judgments "on the ...
, and not risk management (those decision processes involving analysis of regulatory, legal, social and economic considerations related to the risks being studied).
History
Initially, in the 1980s, the IRIS program established a database of human health assessments about the impacts of chemicals in the environment. EPA created the database to provide a consistent approach to risk assessment practices across the various environmental laws that the Agency implemented and enforced. The program was created by the EPA in 1985. Initially, the goal of the program was to foster consistency's in the agency's evaluation of chemicaltoxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
. The IRIS database was first made publicly available in 1987. In 1996, the EPA implemented a new process for building intra-agency consensus and improving efficiency within the IRIS database. The same year, the EPA introduced the IRIS Toxicological Review, which presented the first agency-wide health assessment document. In March 1997, the IRIS database was uploaded to the internet. In 2004, the IRIS process was changed to add an interagency review led by the Office of Management and Budget
The Office of Management and Budget (OMB) is the largest office within the Executive Office of the President of the United States (EOP). OMB's most prominent function is to produce the president's budget, but it also examines agency programs, pol ...
(OMB) and to place a higher emphasis on outside peer review
Peer review is the evaluation of work by one or more people with similar competencies as the producers of the work ( peers). It functions as a form of self-regulation by qualified members of a profession within the relevant field. Peer revie ...
of IRIS assessments.
The IRIS program was once again revised in April 2008. Among other things, the newly revised program provided opportunity for research to close data gaps on mission critical chemicals. The following year, the process was changed to streamline a review schedule so that a majority of assessments would be posted to the IRIS database within two years of the start date. In 2013, the EPA announced that it would be making enhancements to the IRIS process in order to improve transparency, efficiency, and the scientific foundation of the agency's assessments. That same year, the program's Toxicological Review documents were restructured to make them more clear, systematic and consistent. In December 2015, the IRIS program released its first ever multi-year agenda, which identified the top chemical assessments the program would focus on in the following years.
IRIS process for developing human health assessments
The IRIS process takes about 26 to 39 months to complete, depending on the complexity of the assessment, with the review part of the process taking between 15 to 24 months. The process begins with what the EPA refers to as the "Planning and Scoping" stage. During this stage, risk assessors will consider the scope and possible legal limitations as well as how the information will be used. After the Planning and Scoping Stage, the EPA uses a seven-step risk assessment review program that starts with a draft assessment and ends in publication on an IRIS website. The review process proceeds as follows: # Complete draft IRIS assessment. The IRIS program conducts literature search and critical study selection, develops evidence tables that summarize the results of these studies. EPA publicly releases assessment protocols which presents the methods for conducting the systematic review. These protocols include a literature search strategy and study selection criteria. It is also during this stage that the agency identifies hazards, selects studies for dose-response assessment, and derives toxicity values. # Internal agency review. The IRIS program shares its draft assessment with the EPA's program and regional offices, identifies any scientific issues, and determines external peer review scope and guidelines. This process typically lasts 60 days. # Interagency science consultation. Other federal agencies including theExecutive Office of the President
The Executive Office of the President (EOP) comprises the offices and agencies that support the work of the president at the center of the executive branch of the United States federal government. The EOP consists of several offices and agenc ...
(i.e. OMB and the Council on Environmental Quality
The Council on Environmental Quality (CEQ) is a division of the Executive Office of the President that coordinates federal environmental efforts in the United States and works closely with agencies and other White House offices on the developm ...
) review the IRIS draft assessment. EPA then provides a specific date for receiving written comments, convenes a meeting to address the issues raised in the comments, and revises the draft accordingly.
# Independent expert peer review. The IRIS program publicly releases its draft assessment on its website as part of an external review process, after which a meeting is held to allow for peer review charge and scientific questions. The IRIS staff may then revise its assessment draft and submit the draft to an external peer review panel organized by a contractor or by the EPA's Science Advisory Board The Science Advisory Board (SAB) is a United States group of independent scientists selected by the Administrator of the U.S. Environmental Protection Agency (EPA). The board provides advice to the agency on the scientific and technical aspects of ...
.
# Revising assessment. The IRIS program evaluates the recommendations provided by the peer review panel and all public comments. IRIS also prepares a written response-to-comment document. After revising its initial assessment, IRIS staff develop a document describing the disposition of peer review and public comments and provides the document as an appendix to its final assessment.
# Final agency review and interagency science discussion. After completing revisions, the IRIS program shares its assessment with the EPA's program and regional offices for final review. For the interagency part of this stage, EPA provides federal agencies with a final draft of its assessment and related materials, and provides a specific date for receiving written comments. During this stage, the EPA's internal review and the interagency review occur simultaneously.
# Final Assessment. The IRIS program completes its assessment and posts the final product on the IRIS website along with related material, including the Toxicological Review document, IRIS summary and appendices.
Relationship with environmental impact assessments
The IRIS opens a draft review for experts to review in order to assess the course of action needed for the corresponding issue. Action is taken by legislative bodies after assessing the risk and the level of action needed to be taken for the corresponding issue. The relationship between IRIS and anenvironmental impact assessment
Environmental Impact assessment (EIA) is the assessment of the environmental consequences of a plan, policy, program, or actual projects prior to the decision to move forward with the proposed action. In this context, the term "environmental imp ...
(EIA) is that IRIS provides a database used in the EIA process. External parties (scientists, scholars, legislation) make decisions based on the IRIS database. Support for these decisions comes from backing from programs such as Strategic environmental assessment (SEA) and OMB.
References
External links
Integrated Risk Information System
- EPA
Office of Management and Budget; Information and Regulatory affairs
Environmental toxicology Hazard analysis Risk analysis United States Environmental Protection Agency {{Environment-stub